Abstract
Aim
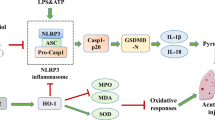
We demonstrate the effect of PDE5 inhibitors in cases of acute lung injury via the relationship between cGMP/NO and the TLR4-NF-κB-NLRP3 pathway.
Materials and Methods
This study was performed with 30 male Wistar albino rats. Lipopolysaccharide (LPS) was administered intratracheally to the rats and acute lung injury (ALI) was induced. Twelve hours after LPS administration, avanafil, prepared at suitable doses according to the body weights of the animals, was administered by oral gavage. Lung tissue samples of all groups were examined histopathologically and by immunochemical staining (IL-1β, iNOS, TLR4, and NF-κB). The iNOS, NLRP3, and IL-1B mRNA expression levels in the lung tissues were measured by RT-PCR. The left upper lobes of the rat lungs were dried at 70 °C for 48 h and lung water content was calculated.
Result
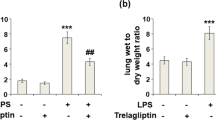
Statistically significant increases in iNOS, NLRP3, and IL-1β mRNA expressions were observed in the rats with ALI compared to the healthy controls (p < 0.0001). Those increased expressions were reduced at both doses of avanafil (p < 0.0001). This reduction was found to be greater at 20 mg/kg (p < 0.0001). IL-1β, iNOS, TLR4, and NF-κB immunopositivity was moderate/severe in the ALI group and mild in the group with ALI + avanafil at 20 mg/kg (p < 0.05). When the wet/dry lung ratios were calculated, a statistically significant increase was seen in the ALI group compared to the healthy rats (p < 0.05). That increase was decreased with both avanafil doses (p < 0.05).
Conclusion
We suggest that avanafil may prevent the progression of ALI and be effective in its treatment. We hope that this study will be supported by future clinical studies to yield a new indication for avanafil.





Similar content being viewed by others
6. References
Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E et al (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307(23):2526–2533. https://doi.org/10.1001/jama.2012.5669
Ashbaugh D, Boyd Bigelow D, Petty T, Levine B (1967) Acute respiratory distress in adults. Lancet 290:319–323. https://doi.org/10.1016/S0140-6736(67)90168-7
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M et al (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693. https://doi.org/10.1056/NEJMoa050333
Goss CH, Brower RG, Hudson LD, Rubenfeld GD (2003) Incidence of acute lung injury in the United States. Crit Care Med 31:1607–1611. https://doi.org/10.1097/01.CCM.0000063475.65751.1D
Torbic H, Krishnan S, Harnegie MP, Duggal A (2021) Neuromuscular blocking agents for ARDS: a systematic review and meta-analysis. Respir Care 66:120–128. https://doi.org/10.4187/respcare.07849
Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R et al (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354:1671–1684. https://doi.org/10.1056/NEJMoa051693
Bischoff E (2004) Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res 16:S11–S14. https://doi.org/10.1038/sj.ijir.3901208
Geraets L, Haegens A, Weseler AR, Brauers K, Vernooy JHJ, Wouters EFM et al (2010) Inhibition of acute pulmonary and systemic inflammation by 1,7-dimethylxanthine. Eur J Pharmacol 629:132–139. https://doi.org/10.1016/j.ejphar.2009.11.064
Mokry J, Mokra D, Nosalova G, Beharkova M, Feherova Z (2008) Influence of selective inhibitors of phosphodiesterase 3 and 4 on cough and airway reactivity. J Physiol Pharmacol 59(Suppl 6):473–482
Mokry J, Urbanova A, Medvedova I, Kertys M, Mikolka P, Kosutova P et al (2017) Effects of tadalafil (PDE5 inhibitor) and roflumilast (PDE4 inhibitor) on airway reactivity and markers of inflammation in ovalbumin-induced airway hyperresponsiveness in guinea pigs. J Physiol Pharmacol 68:721–730
Ahmed WS, Geethakumari AM, Biswas KH (2021) Phosphodiesterase 5 (PDE5): structure-function regulation and therapeutic applications of inhibitors. Biomed Pharmacother 134:111128. https://doi.org/10.1016/j.biopha.2020.111128
Kosutova P, Mikolka P, Balentova S, Kolomaznik M, Adamkov M, Mokry J et al (2018) Effects of phosphodiesterase 5 inhibitor sildenafil on the respiratory parameters, inflammation and apoptosis in a saline lavage-induced model of acute lung injury. J Physiol Pharmacol. https://doi.org/10.26402/jpp.2018.5.15
Laxmi V, Gupta R, Bhattacharya SK, Ray A, Gulati K (2019) Inhibitory effects of sildenafil and tadalafil on inflammation, oxidative stress and nitrosative stress in animal model of bronchial asthma. Pharmacol Reports 71:517–521. https://doi.org/10.1016/j.pharep.2019.02.008
Lubamba B, Huaux F, Lebacq J, Marbaix E, Dhooghe B, Panin N et al (2012) Immunomodulatory activity of vardenafil on induced lung inflammation in cystic fibrosis mice. J Cyst Fibros 11:266–273. https://doi.org/10.1016/j.jcf.2012.03.003
Dawn B, Bollı R (2002) Role of nitric oxide in myocardial preconditioning. Ann N Y Acad Sci 962:18–41. https://doi.org/10.1111/j.1749-6632.2002.tb04053.x
Teng P, Liu H-L, Deng Z-S, Shi Z-B, He Y-M, Feng L-L et al (2011) Synthesis and biological evaluation of unique stereodimers of sinomenine analogues as potential inhibitors of NO production. Bioorg Med Chem 19:3096–3104. https://doi.org/10.1016/j.bmc.2011.04.006
Mehta S (2005) The effects of nitric oxide in acute lung injury. Vascul Pharmacol 43:390–403. https://doi.org/10.1016/j.vph.2005.08.013
Hosseinian N, Cho Y, Lockey RF, Kolliputi N (2015) The role of the NLRP3 inflammasome in pulmonary diseases. Ther Adv Respir Dis 9(4):188–197. https://doi.org/10.1177/1753465815586335
Xu W, Wang X, Jin J, Zou Q, Wu L, Lv T et al (2019) Inhibition of GGPPS1 attenuated LPS-induced acute lung injury and was associated with NLRP3 inflammasome suppression. Am J Physiol Cell Mol Physiol 316:L567–L577. https://doi.org/10.1152/ajplung.00190.2018
Tang J, Xu L, Zeng Y, Gong F (2021) Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol 91:107272. https://doi.org/10.1016/j.intimp.2020.107272
Cui H, Zhang Q (2021) Dexmedetomidine ameliorates lipopolysaccharide-induced acute lung injury by inhibiting the PI3K/Akt/FoxO1 signaling pathway. J Anesth 35:394–404. https://doi.org/10.1007/s00540-021-02909-9
Keskin H, Keskin F, Tavaci T, Halici H, Yuksel TN, Ozkaraca M et al (2021) Neuroprotective effect of roflumilast under cerebral ischaemia/reperfusion injury in juvenile rats through NLRP-mediated inflammatory response inhibition. Clin Exp Pharmacol Physiol 48:1103–1110. https://doi.org/10.1111/1440-1681.13493
Zhao C, Hu L, He X, Li L, Yin M, Tettey AT et al (2022) TPN171H alleviates pulmonary hypertension via inhibiting inflammation in hypoxia and monocrotaline-induced rats. Vascul Pharmacol 145:107017. https://doi.org/10.1016/j.vph.2022.107017
Flores-Costa R, Alcaraz-Quiles J, Titos E, López-Vicario C, Casulleras M, Duran-Güell M et al (2018) The soluble guanylate cyclase stimulator IW-1973 prevents inflammation and fibrosis in experimental non-alcoholic steatohepatitis. Br J Pharmacol 175:953–967. https://doi.org/10.1111/bph.14137
Schwabl P, Brusilovskaya K, Supper P, Bauer D, Königshofer P, Riedl F et al (2018) The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci Rep 8:9372. https://doi.org/10.1038/s41598-018-27656-y
Flores-Costa R, Duran-Güell M, Casulleras M, López-Vicario C, Alcaraz-Quiles J, Diaz A et al (2020) Stimulation of soluble guanylate cyclase exerts antiinflammatory actions in the liver through a VASP/NF-κB/NLRP3 inflammasome circuit. Proc Natl Acad Sci 117:28263–28274. https://doi.org/10.1073/pnas.2000466117
Huyut Z, Bakan N, Yıldırım S, Alp HH (2018) Effects of the phosphodiesterase-5 (PDE-5) ınhibitors, avanafil and zaprinast, on bone remodeling and oxidative damage in a rat model of glucocorticoid-ınduced osteoporosis. Med Sci Monit Basic Res 24:47–58. https://doi.org/10.12659/MSMBR.908504
Wu P, Yan H, Qi J, Jia W, Zhang W, Yao D et al (2020) L6H9 attenuates LPS-induced acute lung injury in rats through targeting MD2. Drug Dev Res 81:85–92. https://doi.org/10.1002/ddr.21607
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Goldring JPD (2019) Measuring protein concentration with absorbance, lowry, bradford coomassie blue, or the smith bicinchoninic acid assay before electrophoresis. Methods Mol Biol 1855:31–39. https://doi.org/10.1007/978-1-4939-8793-1_3
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and ınnate ımmunity. Cell 124:783–801. https://doi.org/10.1016/j.cell.2006.02.015
O’Neill LAJ, Bryant CE, Doyle SL (2009) Therapeutic targeting of toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev 61:177–197. https://doi.org/10.1124/pr.109.001073
Li Q, Verma IM (2002) NF-κB regulation in the immune system. Nat Rev Immunol 2:725–734. https://doi.org/10.1038/nri910
Liu S, Chen Q, Liu J, Yang X, Zhang Y, Huang F (2018) Sinomenine protects against E.coli-induced acute lung injury in mice through Nrf2-NF-κB pathway. Biomed Pharmacother 107:696–702. https://doi.org/10.1016/j.biopha.2018.08.048
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D et al (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791. https://doi.org/10.4049/jimmunol.0901363
Jo E-K, Kim JK, Shin D-M, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13:148–159. https://doi.org/10.1038/cmi.2015.95
Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV et al (2014) Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol 192:5974–5983. https://doi.org/10.4049/jimmunol.1400368
Wen JJ, Cummins C, Radhakrishnan RS (2020) Sildenafil recovers burn-induced cardiomyopathy. Cells 9:1393. https://doi.org/10.3390/cells9061393
Fan Chung K (2006) Phosphodiesterase inhibitors in airways disease. Eur J Pharmacol 533:110–117. https://doi.org/10.1016/j.ejphar.2005.12.059
Zhang C, Wang X, Wang C, He C, Ma Q, Li J et al (2021) Qingwenzhike prescription alleviates acute lung injury induced by LPS via inhibiting TLR4/NF-kB pathway and NLRP3 inflammasome activation. Front Pharmacol 12:790072. https://doi.org/10.3389/fphar.2021.790072
Wagenaar GTM, Hiemstra PS, Gosens R (2015) Therapeutic potential of soluble guanylate cyclase modulators in neonatal chronic lung disease. Am J Physiol Cell Mol Physiol 309:L1037–L1040. https://doi.org/10.1152/ajplung.00333.2015.
Essayan DM (2001) Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol 108:671–680. https://doi.org/10.1067/mai.2001.119555
Sharma R (2007) Novel phosphodiesterase-5 inhibitors: current indications and future directions. Indian J Med Sci 61:667–679
Straub AC, Beuve A (2021) A primer for measuring cGMP signaling and cGMP-mediated vascular relaxation. Nitric Oxide 117:40–45. https://doi.org/10.1016/j.niox.2021.09.008
Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S et al (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52:375–414
Chang J, Ding Y, Zhou Z, Nie H-G, Ji H-L (2018) Transepithelial fluid and salt re-absorption regulated by cGK2 signals. Int J Mol Sci 19:881. https://doi.org/10.3390/ijms19030881
Hou Y, Li J, Ding Y, Cui Y, Nie H (2022) Luteolin attenuates lipopolysaccharide-induced acute lung injury/acute respiratory distress syndrome by activating alveolar epithelial sodium channels via cGMP/PI3K pathway. J Ethnopharmacol 282:114654. https://doi.org/10.1016/j.jep.2021.114654
Wang Q, Zheng X, Cheng Y, Zhang Y-L, Wen H-X, Tao Z et al (2014) Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na, K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J Immunol 192:3765–3777. https://doi.org/10.4049/jimmunol.1302421
Sproston NR, El Mohtadi M, Slevin M, Gilmore W, Ashworth JJ (2018) The effect of C-reactive protein ısoforms on nitric oxide production by U937 monocytes/macrophages. Front Immunol 9:1500. https://doi.org/10.3389/fimmu.2018.01500
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2:907–916. https://doi.org/10.1038/ni1001-907
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
PA. contributed to the literature review, design, writing of the main manuscript. ZBAM.contributed to the literature review, data collection and/or processing. SKU.contributed to the design, analysis and/or interpretation. HH.contributed to the analysis and/or interpretation, and preparation of figures 1-4. NA. contributed to the preparation of tables 1 and 2. ASM.contributed to the data collection and/or processing. BM. contributed to the data collection and/or processing. EC. contributed to the critical review. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
The study was approved by the Local Ethics Committee of Ataturk University (Local Ethics Council of Animal Experiments, Protocol No: 09.07.2021 issue number E-42190979–000-2100184052).
Informed Consent
Not Applicable.
Research Involving Animal Rights
All animal procedures and experiments were performed according to national guidelines for laboratory animals’ use and care and the European Community Council Directives (2010/63/EU).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aydin, P., Magden, Z.B.A., Uzuncakmak, S.K. et al. Avanafil as a Novel Therapeutic Agent Against LPS-Induced Acute Lung Injury via Increasing CGMP to Downregulate the TLR4-NF-κB-NLRP3 Inflammasome Signaling Pathway. Lung 200, 561–572 (2022). https://doi.org/10.1007/s00408-022-00564-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00564-9