Abstract
Purpose
Vitronectin (VTN), a multifunctional glycoprotein, is involved in various biological and pathological processes. The purpose of this study was to explore the effect of VTN on mesenchymal-epithelial transition (MET) of pulmonary fibroblast cells.
Methods
Lentivirus encoding for VTN-specific shRNA was constructed and infected into the cultured fibroblast WI-38 cells. Real-time PCR and Western blot were applied to examine the expression of VTN in WI-38 cells. MTT assay was used to assess cell proliferation. Western blot was conducted to examine the expression of MET-related and apoptosis-related proteins.
Results
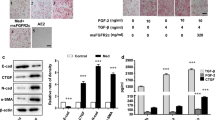
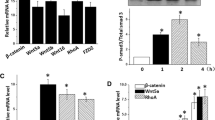
The knockdown of VTN significantly inhibited the growth of WI-38 cells compared to the control group. Meanwhile, knockdown of VTN remarkably increased the expression of Bax and Caspase 3 compared with the control group. Furthermore, knockdown of VTN significantly promoted the expression of E-cadherin in comparison to control group.
Conclusions
Knockdown of VTN promoted the expression of apoptosis-related factors, meanwhile, facilitated the MET process of fibroblast cells by regulating the expression of relevant factors. In sum, VTN performed a potential regulator in cell growth and MET of pulmonary fibroblast cells, which can be considered as a potential target for diagnose and therapy of relevant diseases.



Similar content being viewed by others
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hayman EG, Engvall E, A’Hearn E, Barnes D, Pierschbacher M, Ruoslahti E (1982) Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol 95(1):20–23. https://doi.org/10.1083/jcb.95.1.20
Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E (1985) Vitronectin–a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res 160(2):245–258. https://doi.org/10.1016/0014-4827(85)90173-9
Jenne D, Stanley KK (1985) Molecular cloning of S-protein, a link between complement, coagulation and cell-substrate adhesion. EMBO J 4(12):3153–7
Preissner KT (1989) The role of vitronectin as multifunctional regulator in the hemostatic and immune systems. Blut 59(5):419–431. https://doi.org/10.1007/BF00349063
Singh B, Blom AM, Unal C, Nilson B, Morgelin M, Riesbeck K (2010) Vitronectin binds to the head region of Moraxella catarrhalis ubiquitous surface protein A2 and confers complement-inhibitory activity. Mol Microbiol 75(6):1426–1444. https://doi.org/10.1111/j.1365-2958.2010.07066.x
Singh B, Su YC, Riesbeck K (2010) Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion. Mol Microbiol 78(3):545–560. https://doi.org/10.1111/j.1365-2958.2010.07373.x
Huang Y, Zhang W, Yu F, Gao F (2017) The cellular and molecular mechanism of radiation-induced lung injury. Med Sci Monit 23:3446–3450. https://doi.org/10.12659/msm.902353
Giridhar P, Mallick S, Rath GK, Julka PK (2015) Radiation induced lung injury: prediction, assessment and management. Asian Pac J Cancer Prev 16(7):2613–2617. https://doi.org/10.7314/apjcp.2015.16.7.2613
Huang Y, Liu W, Liu H, Yang Y, Cui J, Zhang P et al (2014) Grape seed pro-anthocyanidins ameliorates radiation-induced lung injury. J Cell Mol Med 18(7):1267–1277. https://doi.org/10.1111/jcmm.12276
Cai XW, Shedden K, Ao X, Davis M, Fu XL, Lawrence TS et al (2010) Plasma proteomic analysis may identify new markers for radiation-induced lung toxicity in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 77(3):867–876. https://doi.org/10.1016/j.ijrobp.2010.01.038
Carpagnano GE, Kharitonov SA, Wells AU, Pantelidis P, Du Bois RM, Barnes PJ (2003) Increased vitronectin and endothelin-1 in the breath condensate of patients with fibrosing lung disease. Respiration 70(2):154–160. https://doi.org/10.1159/000070062
Pohl WR, Conlan MG, Thompson AB, Ertl RF, Romberger DJ, Mosher DF et al (1991) Vitronectin in bronchoalveolar lavage fluid is increased in patients with interstitial lung disease. Am Rev Respir Dis 143(6):1369–1375. https://doi.org/10.1164/ajrccm/143.6.1369
Salazar-Pelaez LM, Abraham T, Herrera AM, Correa MA, Ortega JE, Pare PD et al (2015) Vitronectin expression in the airways of subjects with asthma and chronic obstructive pulmonary disease. PLoS ONE 10(3):e0119717. https://doi.org/10.1371/journal.pone.0119717
Taliana L, Evans MD, Ang S, McAvoy JW (2006) Vitronectin is present in epithelial cells of the intact lens and promotes epithelial mesenchymal transition in lens epithelial explants. Mol Vis 12:1233–1242
Colella B, Faienza F, Di Bartolomeo S (2019) EMT regulation by autophagy: a new perspective in glioblastoma biology. Cancers (Basel). https://doi.org/10.3390/cancers11030312
Becerril C, Montano M, Cisneros J, Mendoza-Milla C, Pardo A, Ortiz-Quintero B et al (2021) Mesenchymal-epithelial transition in fibroblasts of human normal lungs and interstitial lung diseases. Biomolecules. https://doi.org/10.3390/biom11030378
Zhu W, Liu Y, Hu K, Li W, Chen J, Li J et al (2014) Vitronectin promotes cell growth and inhibits apoptotic stimuli in a human hepatoma cell line via the activation of caspases. Can J Physiol Pharmacol 92(5):363–368. https://doi.org/10.1139/cjpp-2014-0032
Shen T-L, Liu M-N, Zhang Q, Feng W, Yu W, Fu X-L et al (2018) The positive role of vitronectin in radiation induced lung toxicity: the in vitro and in vivo mechanism study. J Transl Med 16(1):100. https://doi.org/10.1186/s12967-018-1474-y
Wei F, Tang L, He Y, Wu Y, Shi L, Xiong F et al (2018) BPIFB1 (LPLUNC1) inhibits radioresistance in nasopharyngeal carcinoma by inhibiting VTN expression. Cell Death Dis 9(4):432. https://doi.org/10.1038/s41419-018-0409-0
Preissner KT, Jenne D (1991) Vitronectin: a new molecular connection in haemostasis. Thromb Haemost 66(2):189–194
Singh B, Janardhan KS, Kanthan R (2005) Expression of angiostatin, integrin alphavbeta3, and vitronectin in human lungs in sepsis. Exp Lung Res 31(8):771–782. https://doi.org/10.1080/01902140500324901
Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E (2007) Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol 179(10):7079–7086. https://doi.org/10.4049/jimmunol.179.10.7079
Sen R, Baltimore D (1986) Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46(5):705–716. https://doi.org/10.1016/0092-8674(86)90346-6
Hoesel B, Schmid JA (2013) The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 12:86. https://doi.org/10.1186/1476-4598-12-86
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI et al (2016) Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365(3):495–506. https://doi.org/10.1007/s00441-016-2464-0
Sen A, Ta M (2020) Altered adhesion and migration of human mesenchymal stromal cells under febrile temperature stress involves NF-kappabeta pathway. Sci Rep 10(1):4473. https://doi.org/10.1038/s41598-020-61361-z
Palma CDS, Grassi ML, Thomé CH, Ferreira GA, Albuquerque D, Pinto MT et al (2016) Proteomic analysis of epithelial to mesenchymal transition (EMT) reveals cross-talk between SNAIL and HDAC1 proteins in breast cancer cells. Mol Cell Proteomics 15(3):906–917. https://doi.org/10.1074/mcp.M115.052910
Guan X (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B 5(5):402–418. https://doi.org/10.1016/j.apsb.2015.07.005
Sun J, Li Q, Lian X, Zhu Z, Chen X, Pei W et al (2019) MicroRNA-29b mediates lung mesenchymal-epithelial transition and prevents lung fibrosis in the silicosis model. Mol Ther Nucleic Acids 14:20–31. https://doi.org/10.1016/j.omtn.2018.10.017
Funding
This work was supported by the National Natural Science Foundation of China (No.81572950), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (No.20161433), and Shanghai Shen Kang Hospital Development Center Clinical Science and Technology Innovation Project (No.SHDC12017103).
Author information
Authors and Affiliations
Contributions
XC proposed the concept and the design of experimental scheme of this study. YZ completed the related experiments in this study. JY collated and analyzed the experimental data. ML and QZ cooperated to complete the manuscript. XC reviewed the experimental data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, Y., Yu, J., Liu, M. et al. Analyzing the Effect of Vitronectin on Cell Growth and Mesenchymal-Epithelial Transition of Pulmonary Fibroblast Cells. Lung 199, 389–394 (2021). https://doi.org/10.1007/s00408-021-00467-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-021-00467-1