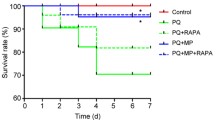
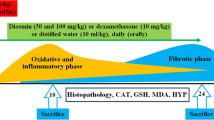
Abstract
Purpose
Patients with pulmonary fibrosis often exhibit reduced lung function and diminished health-related quality of life. Studies have shown that paraquat-induced, extrapulmonary, acute lung injury affects the metabolic profile of glycogen content in different tissues. The purpose of the present study was to investigate whether the process of pulmonary fibrosis induced by continuous exposure to the toxic herbicide paraquat or by a local insult from bleomycin affects the glycogen content in tissues.
Methods
In the paraquat experiment, Wistar rats (n = 5 per group) received either saline (controls) or an intraperitoneal injection of a paraquat solution (7.0 mg/kg; experimental group) once a week for 4 weeks. In the bleomycin experiment, Balb/c mice (n = 5 per group) received either saline (controls) or 6.25 U/kg of bleomycin through intratracheal instillation in single dose (experimental group). Glycogen content in different tissues (mg/g tissue) was measured using the anthrone reagent. The lungs submitted to histopathological and quantitative analyses of fibrosis.
Results
Paraquat-induced fibrosis led to lower glycogen content in the gastrocnemius muscle (2.7 ± 0.1 vs. 3.4 ± 0.1; 79 %) compared with the controls, whereas no changes in glycogen content were found in the diaphragm or heart. Bleomycin-induced fibrosis led to lower glycogen content in the diaphragm (0.43 ± 0.02 vs. 0.79 ± 0.09, 54 %), gastrocnemius muscle (0.62 ± 0.11 vs. 1.18 ± 0.06, 52 %), and heart (0.68 ± 0.11 vs. 1.39 ± 0.1, 49 %) compared with the controls (p < 0.05). Moreover, the area of fibrous connective tissue (μm2) in the lungs was significantly increased in paraquat-induced fibrosis (3,463 ± 377 vs. 565 ± 89) and bleomycin-induced fibrosis (3,707 ± 433.9 vs. 179 ± 51.28) compared with the controls.
Conclusions
The findings suggest that the effects of fibrogenesis in the lungs are not limited to local alterations but also lead to a reduction in glycogen content in the heart and other muscles. This reduction could partially explain the impaired muscle performance found in patients with pulmonary fibrosis.




Similar content being viewed by others
References
Shi W, Xu J, Warburton D (2009) Development, repair and fibrosis: what is common and why it matters. Respirology 14:656–665. doi:10.1111/j.1440-1843.2009.01565.x
Kottmann RM, Hogan CM, Phipps RP, Sime PJ (2009) Determinants of initiation and progression of idiopathic pulmonary fibrosis. Respirology 14:917–933. doi:10.1111/j.1440-1843.2009.01624.x
du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, King TE Jr (2011) Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med 183:1231–1237. doi:10.1164/rccm.201007-1179OC
Mura M, Ferretti A, Ferro O, Zompatori M, Cavalli A, Schiavina M, Fabbri M (2006) Functional predictors of exertional dyspnea, 6-min walking distance and HRCT fibrosis score in idiopathic pulmonary fibrosis. Respiration 73:495–502. doi:10.1159/000089656
Hsia CC (1999) Cardiopulmonary limitations to exercise in restrictive lung disease. Med Sci Sports Exerc 31:S28–S32
Prasse A, Müller-Quernheim J (2009) Non-invasive biomarkers in pulmonary fibrosis. Respirology 14:788–795. doi:10.1111/j.1440-1843.2009.01600.x
Borges EL, Pinheiro MB, Eleto-Silva A, Caliari MV, Rodrigues-Machado MG (2009) Glycogen content is affected differently in acute pulmonary and extrapulmonary lung injury. Hum Exp Toxicol 28:583–590. doi:10.1177/0960327109106998
Rodrigues-Machado MG, Silva GC, Pinheiro MB, Caliari MV, Borges EL (2010) Effects of sepsis-induced acute lung injury on glycogen content in different tissues. Exp Lung Res 36:302–306. doi:10.3109/01902141003609983
Silva MF, Saldiva PH (1998) Paraquat poisoning: an experimental model of dose-dependent acute lung injury due to surfactant dysfunction. Braz J Med Biol Res 31:445–450
Lacerda ACR, Rodrigues-Machado MG, Mendes PL, Novaes RD, Carvalho GM, Zin WA, Gripp F, Coimbra CC (2009) Paraquat (PQ)-induced pulmonary fibrosis increases exercise metabolic cost, reducing aerobic performance in rats. J Toxicol Sci 34:671–679. doi:10.2131/jts.34.671
Dasta JF (1978) Paraquat poisoning: a review. Am J Hosp Pharm 35:1368–1372
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295:L379–L399. doi:10.1152/ajplung.00010.2008
Moore BB, Hogaboam CM (2008) Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294:L152–L160. doi:10.1152/ajplung.00313.2007
Satomi Y, Sakaguchi K, Kasahara Y, Akahori F (2007) Novel and extensive aspects of paraquat-induced pulmonary fibrogenesis: comparative and time-course microarray analyses in fibrogenic and non-fibrogenic rats. J Toxicol Sci 32:529–553. doi:10.2131/jts.32.529
Satomi Y, Tsuchiya W, Miura D, Kasahara Y, Akahori F (2006) DNA microarray analysis of pulmonary fibrosis three months after exposure to paraquat in rats. J Toxicol Sci 31:345–350. doi:10.2131/jts.31.345
Satomi Y, Tsuchiya W, Mihara K, Ota M, Kasahara Y, Arahori F (2004) Gene expression analysis of the lung following paraquat administration in rats using DNA. J Toxicol Sci 29:91–100. doi:10.2131/jts.29.91
Rocco PR, Negri EM, Kurtz PM, Vasconcellos FP, Silva GH, Capelozzi VL, Romero PV, Zin WA (2001) Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med 164:1067–1071
Akahori F, Oehme FW (1983) Inhibition of collagen synthesis as a treatment for paraquat poisoning. Vet Hum Toxicol 25:321–327
Prata LO, Oliveira FM, Ribeiro TM, Almeida PW, Cardoso JA, Rodrigues-Machado Mda G, Caliari MV (2012) Exercise attenuates pulmonary injury in mice with bleomycin-induced pulmonary fibrosis. Exp Biol Med 237:873–883. doi:10.1258/ebm.2012.011334
Sáez MJ, Lagunas R (1976) Determination of intermediary metabolites in yeast. Critical examination of the effect of sampling conditions and recommendations for obtaining true levels. Mol Cell Biochem 13:73–78
Hassid WZ, Abraham S (1957) Determination of glycogen with anthrone reagent. In: Abelson JN, Simon MI, Colowisch SP, Kaplan NO (eds) Methods in enzymology. Section I: carbohydrates, vol 3. Academic Press, New York, pp 35–36
Chua F, Gaudle J, Laurent GJ (2005) Pulmonary fibrosis searching for model answers. Am J Respir Cell Mol Biol 33:9–13. doi:10.1165/rcmb.2005-0062TR
Moeller A, Ask K, Warburton D, Gauldie J, Kolb M (2008) The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40:362–382. doi:10.1016/j.biocel.2007.08.011
Spruit MA, Janssen DJ, Franssen FM, Wouters EF (2009) Rehabilitation and palliative care in lung fibrosis. Respirology 14:781–787. doi:10.1111/j.1440-1843.2009.01599.x
Nishiyma O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, Nishimura K (2005) Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med 99:408–414. doi:10.1016/j.rmed.2004.09.005
Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK (2005) Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax 60:588–594. doi:10.1136/thx.2004.035220
Tzanakis N, Samiou M, Lambiri I, Antoniou K, Siafakas N, Bouros D (2005) Evaluation of health-related quality-of-life and dyspnea scales in patients with idiopathic pulmonary fibrosis. Correlation with pulmonary function tests. Eur J Intern Med 16:105–112. doi:10.1016/j.ejim.2004.09.013
De Vries J, Kessels BL, Drent M (2001) Quality of life of idiopathic pulmonary fibrosis patients. Eur Resp J 17:954–961
Martinez TY, Pereira CAC, Santos ML, Ciconnelli RM, Guimaraes SM, Martinez JAB (2000) Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with idiopathic pulmonary fibrosis. Chest 117:1627–1632
Zocrato LB, Capettini LS, Rezende BA, Silva JF, Rodrigues-Machado MG, Cortes SF, Lemos VS (2010) Increased expression of endothelial iNOS accounts for hyporesponsiveness of pulmonary artery to vasoconstrictors after paraquat poisoning. Toxicol In Vitro 24:1019–1025. doi:10.1016/j.tiv.2009.12.003
Elenkov IJ, Chrousos GP (2006) Stress system—organization, physiology and immunoregulation. NeuroImmunoModulation 13:257–267. doi:10.1159/000104853
Heindl S, Lehnert M, Criée CP, Hasenfuss G, Andreas S (2001) Market sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med 164:597–601
Conflict of interest
The authors declare no competing interests.
Ethical standards
The authors declare that the experiments comply with the current laws of the country in which they were performed. All experiments were performed in compliance with the Ethical Principles in Animal Experimentation adopted by the local Ethics Committee on Animal Experimentation (CETEA/UFMG process No. 60/2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borges, E.L., de Barros Pinheiro, M., Prata, L.O. et al. Effect of Lung Fibrosis on Glycogen Content in Different Extrapulmonary Tissues. Lung 192, 125–131 (2014). https://doi.org/10.1007/s00408-013-9539-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9539-4