Abstract
Recent advances in our understanding of voltage-gated sodium channels (NaVs) lead to the rational hypothesis that drugs capable of selective blockade of NaV subtypes may be a safe and effective strategy for the treatment of unwanted cough. Among the nine NaV subtypes (NaV1.1–NaV1.9), the afferent nerves involved in initiating cough, in common with nociceptive neurons in the somatosensory system, express mainly NaV1.7, NaV1.8, and NaV1.9. Although knowledge about the effect of selectively blocking these channels on the cough reflex is limited, their biophysical properties indicate that each may contribute to the hypertussive and allotussive state that typifies subacute and chronic nonproductive cough.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
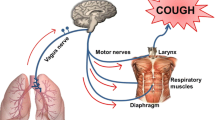
Cough can be initiated from activation of peripheral afferent (sensory) nerves or initiated from processes occurring within the central nervous system (CNS) independently of afferent nerves. Cough initiated from the CNS can be subcategorized as voluntary cough or psychogenic cough. Cough initiated from the periphery can be subcategorized into the protective cough reflex vital for airway defense on one hand, and the irritating, itchy, urge-to-cough that serves essentially no useful purpose, on the other. From a therapeutic perspective, it is logical to focus attention on psychogenic cough and the afferent initiated nonproductive urge-to-cough sensations and avoid inhibiting protective cough or voluntary cough. With respect to inhibiting cough initiated by activation of peripheral sensory nerves, the voltage-gated sodium channels (NaVs) are particularly attractive therapeutic targets.
Cough associated with respiratory viral infections, respiratory diseases, and esophageal reflux are initiated by peripheral afferent nerves. Often the cough associated with these disorders develops into an itchy, nonproductive cough. These sensations and the resulting nonproductive coughing can be mimicked by inhalation of low concentrations of agents known to stimulate vagal afferent C-fibers. Yet, there is little evidence for the existence of a truly effective peripherally acting antitussive drug.
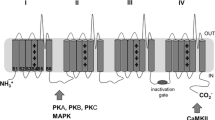
NaVs are a sine qua non of action potential discharge [1]. They provide the current for the action potential spike, and they are determinant in voltage thresholds, spike frequency, and conduction. A breakthrough in our understanding of NaVs came with the unraveling of their molecular biology [2]. The NaVs comprise large α subunits with four homologous domains and two noncovalently linked β subunits. The α subunits are the pore-forming proteins and are encoded by nine distinct genes. The channels formed are referred to NaV1.1–1.9. These channels can be blocked nonselectively with the class of drugs known as local anesthetics. They are “local” because systemic blockade of all NaVs is lethal, limiting their utility in the treatment of visceral diseases.
Proof of Concept
Local anesthetics have been tried in proof-of-concept trials for NaV blockade in cough. Although local treatment with lidocaine inhibits cough evoked by mechanical stimulation of the larynx/trachea, and nebulized lidocaine causes a short-lasting partial inhibition of cough induced by inhaled capsaicin [3], it has thus far shown to be of little use in the treatment of pathological cough. Case reports show the antitussive efficacy of nebulized lidocaine in patients with chronic cough [4, 5], but in a large study examining cough in COPD, lidocaine was not effective [6]. An oral formulation of benzonatate, a derivative of the local anesthetic procaine, is used for the treatment of cough, but the evidence of its effectiveness is scanty [7]. The lack of impressive efficacy of nebulized lidocaine or oral benzonatate should not, however, be taken as a lack of “proof-of-concept” for NaVs in the treatment of cough. Local anesthetics have relatively low affinities for NaVs, and it is likely that at the doses that can be administered safely, they only weakly and briefly inhibit NaVs in the afferent C-fiber and A-fiber terminals involved in cough. More potent nonselective NaVs are available, but severe toxicity prevents their use in humans. For example, tetrodotoxin (TTX) blocks seven of the nine NaVs (all except NaV1.5, 1.8 and 1.9) with ~1000 times greater potency than lidocaine. Systemic administration of this toxin however leads to rapid death that is likely secondary the paralysis of respiratory muscles.
All peripherally acting stimuli for cough must first interact with afferent nerve terminals to cause a membrane depolarization. This initial stimulus-dependent depolarization is referred to as a “generator potential” (Fig. 1). The nature of the activating stimuli for the Aδ-fiber “cough receptor” terminal in the large airways appears to be relatively limited [8]. In guinea pigs, the A-fiber cough receptor is stimulated by punctuate mechanical perturbation of the epithelium and by rapid decreases in pH; it is by in large not stimulated by mediators of inflammation. The vagal airway afferent C-fibers, by contrast, have a much more promiscuous activation profile. Depending on the C-fiber subtype, these nerves can be activated by numerous chemicals and inflammatory mediators. In many cases, these stimuli lead to generator potentials by stimulating ionotropic receptors. Stimuli that gate ionotropic receptors include ATP acting via P2X2/3 receptors, 5-HT acting via 5-HT3 receptors, nicotine via nicotinic receptors, and the panoply of irritating substances that can activate TRPV1 or TRPA1 channels. Certain mediators that stimulate G-protein receptors also can lead to generator potentials in vagal afferent C-fibers in the airways. These include bradykinin via B2 receptors and adenosine via A1 and A2A receptors [9–14].
Illustration of the concept of afferent nerve terminal activation. Stimuli act on various receptors and ion channels to cause a membrane depolarization that is referred to as the generator potential. This in turn activates voltage-gated sodium channels (NaV) that are responsible for action potential generation and conduction to the central terminal in the brainstem. The yellow image is an actual guinea pig vagal afferent nerve terminal. The example of a generator potential is actually a depolarizing potential recorded with patch clamp technology at the level of the cell soma (due to technical difficulties, generator potentials have not yet been recorded at vagal afferent nerve terminals)
Generator potentials are of little consequence unless they are of a rate and magnitude sufficient to trigger an action potential. Short of this, generator potentials electronically decline to the resting potential over a time and distance that is based on the time and space constant of the nerve membrane. The triggering of the action potential occurs only when the voltage threshold of NaVs is reached, the channels are opened, and the rush of sodium ion traverses the membrane (Fig. 1). When NaVs in vagal afferent nerves are blocked, action potentials are not generated, and the communication between the innervated organ and CNS is silenced. In other words, blockade of NaVs is a form of “chemical denervation” every bit as effective as surgical sectioning of the nerve. If you silence the communication between the respiratory tract (and perhaps esophagus) and the brainstem, there will be no urge-to-cough initiated from peripheral sources (although voluntary cough and psychogenic cough would be unaffected). In a sense, the proof of concept for NaV blockade in cough can be found in double-lung-transplant patients, where indeed it is not excessive coughing but a lack of coughing that poses the potential problem [15].
Selective NaV Blockade for Cough
The NaV1 subtypes (NaV1.1–NaV1.9) are differentially distributed among neurons, cardiac muscle, and skeletal muscle [2]. For example, the NaV involved in action potential generation in skeletal muscle is largely NaV1.4, the NaV expressed in cardiac myocytes is principally 1.5, and the neurons in the brain express largely NaV1.1, 1.2, 1.3, and 1.6. This differential distribution paves the way for developing selective NaV blockers with therapeutic indexes much greater than can be obtained with nonselective local anesthetics. The question arises, what types of NaVs are expressed by afferent neurons involved in the cough reflex? This important issue has received relatively little experimental attention, but in guinea pigs, the vagal afferent A-fiber cough receptors and C-fiber neurons innervating the respiratory tract were found to express primarily NaV1.7, NaV1.8, and NaV1.9 [16, 17] (Fig. 2). This is potentially very encouraging information, because these channels have a relatively limited expression elsewhere in the body [18]. They are not expressed by skeletal or cardiac muscle and scantly expressed in the CNS. They are strongly expressed in small diameter, presumed nociceptive, neurons in the dorsal root ganglia. This has led to the hypothesis that NaV1.7, 1.8, and 1.9 may play important roles in transmitting pain signals to the brain [19–21]. Accordingly, the past decade has witnessed intensive efforts within the pharmaceutical industry towards discovering selective NaV1.7, 1.8, and 1.9 blockers for the treatment of neuropathic and inflammatory pain. Several companies have developed safe NaV blockers that are in various stages of development [21]. The time is ripe to investigate the potential of these same products for peripheral acting antitussive agents.
NaV1.7
Among the three principal NaVs expressed in airway specific vagal afferent neurons (NaV 1.7, 1.8, and 1.9), NaV1.7 is the only one that is sensitive to blockade with TTX. NaV1.8 and 1.9 are TTX-resistant channels. TTX is effective at blocking action potential conduction in vagal afferent nerve fibers [17, 22]; therefore, it is likely that NaV1.7 plays an important role in this regard as well. This hypothesis was addressed using NaV1.7 shRNA delivered to the vagal sensory neurons via adeno-associated virus (AAV) in vivo [17]. This treatment nearly abolished expression of NaV1.7 without influencing expression of other NaVs in the transfected vagal neurons. In the absence of NaV1.7, the neurons were much less excitable (required a much larger depolarizing stimulus to evoke an action potential), and they were incapable of firing action potentials at high frequencies (Fig. 3). This latter point is relevant in that cough is preferentially triggered by high frequency action potential input to the brainstem [23, 24]. When the vagus nerve was isolated from animals in which the expression of NaV1.7 was silenced, the number of afferent nerve fibers capable of conducting action potentials was substantially inhibited. Thus, inhibiting the expression of NaV1.7 recapitulates many of the effects of TTX on vagal afferent nerves. It was not surprising therefore, that a lack of NaV1.7 was associated with inhibition of C-fiber and Aδ-fiber mediated cough in guinea pigs [17, 25].
Patch clamp recordings of a vagal sensory nodose neurons isolate from a control guinea pig (left) or a guinea pig that was previously treated with NaV1.7shRNA to block expression of NaV1.7 channels (right). a Example of an experiment in which the amount of depolarizing current required to evoke an action potential was determined. In this control neuron 20 pA was required, whereas in the NaV1.7 shRNA treated neuron 80 pA was required. The average ± SEM from 12 experiments is stated below the figures. b The frequency of action potential discharge in response to a supramaximal 1 s depolarizing current was determined; note how the frequency of firing is reduced in the absence of NaV1.7. For details see [17]
Another point making NaV1.7 an attractive target for inflammatory associated cough is the observation that P38 mitogen-activated protein kinase and ERK kinases can lead to channel phosphorylation and a consequent increase in the sodium current density [26]. Inflammation also has been associated with an increase in expression of NaV1.7 [27]. A NaV1.7 blocker, at the right dose, therefore may normalize the hyper-excitable state of afferent terminals at the sites of inflammation. A rare loss-of-function mutation in NaV1.7 leads to a congenital insensitivity to pain, yet with otherwise normal neuronal function and normal sensations to other non-painful stimuli [28], although a recent report indicates that those with this mutation also have a diminished sense of smell [29]. The cough sensitivity in these subjects has not been evaluated.
NaV1.8
In the presence of TTX (that blocks NaV1.1–NaV1.7), depolarizing current leads to a relatively large, fast, inactivating sodium current, in airway-specific sensory neurons that is likely secondary to the gating of NaV1.8. Elegant modeling has indicated that along with NaV1.7, NaV1.8 has a substantive effect on the neurons excitability and capacity for action potential discharge in vagal sensory neurons [30]. Studies in both spinal afferent neurons [31] and in vagal afferent neurons [32] have demonstrated that inflammatory mediators known to increase the excitability of afferent C-fibers and increase cough sensitivity [33] can, by multiple mechanisms, lead to NaV1.8 phosphorylation and an increase in sodium current density. NaV1.8 (as with NaV1.7 and NaV1.9) also appears to be upregulated transcriptionally by inflammatory processes [27]. As with NaV1.7, inhibiting NaV1.8 may therefore normalize C-fibers that are in a hyperexcitable state due to an inflammatory reaction. Consistent with this idea, blocking NaV1.8 has little effect on the response to painful stimuli in healthy animals, but profoundly inhibits the hyperalgesia in response to inflammatory lesions [19, 34]. There is no information on the effect of NaV1.8 blockade specifically on cough associated afferent nerves.
NaV1.9
The other TTX-resistant current in vagal afferent neurons involved in cough is activated at membrane potentials closer to the resting potential, and unlike NaV1.7 and NaV1.8 results in a persistent very slowly inactivating current. The slowly inactivating NaV current is largely gone in neurons from NaV1.9 knockout mice [35]. NaV1.9 is strongly expressed in C-fibers and A-fibers involved in the cough reflex in guinea pigs [16], but there is no information on how this channel modulates cough sensitivity. Once again, we are left with studies on pain sensations for potential analogies [36]. Inflammatory mediators, such as PGE2, shifts the steady-state activation of this channel in a hyperpolarizing direction, which might be expected for a mechanisms that could increase nerve excitability [37]. As with NaV1.8, when NaV1.9 is genetically deleted, there is little change in the response of the healthy mouse to various painful stimuli. However, the hyperalgesia that accompanies the injection of various inflammatory mediators, such as bradykinin and PGE2, is absent in NaV1.9 −/− mice [35]. Certain models of inflammation-induced hyperalgesia are normalized in NaV1.9 −/− mice [35]. Likewise, inflammation associated bladder hyperreactivity is diminished in NaV1.9 −/− mice [38].
Advantages of Targeting NaVs for Cough
Blocking NaVs will decrease the efficacy by which generator potentials lead to action potentials in all afferent nerve terminals. It will also lead to a decrease in the action potential discharge frequency conducted in the vagal afferent nerves to the brain stem. This will be the case irrespective of the nature of the stimuli that induces the generator potential. A decided advantage of NaV blockers in cough is that in theory they will be equally effective at quelling cough evoked by stimuli as disparate as mechanical perturbations, acid, osmotic changes, TRPV1 stimulants, TRPA1 stimulants, bradykinin, ATP, and various inhaled irritants.
NaV blockers could conceivably be administered topically via aerosol, but due to the relatively limited expression of NaV1.7, 1.8, and 1.9, these drugs are likely to be relatively safe given systemically. This could be an advantage when the afferent nerve driving the pathological cough is situated outside the airway wall, e.g., in the nasal mucosa, oral pharynx, or esophagus.
The third advantage of targeting NaVs is based on the studies showing that NaV1.7, 1.8, and 1.9 are all “upregulated” in the presence of inflammation, and specific inflammatory mediators [27]. It is possible that targeting these channels could block the hypertussivity and allotussivity associated with pathological cough without inhibiting the protective cough reflex.
Disadvantages of Targeting NaVs for Cough
Although the NaVs expressed in cough causing afferent nerves have a limited distribution in the body, there is little evidence that subtypes of vagal afferent C-fibers and A-fibers differentially express NaVs. Therefore, if the NaVs were maximally blocked, one would anticipate that the protective cough reflex could be compromised. Based on the limited information available, this is likely to be truer for NaV1.7 than either NaV1.8 or NaV1.9. This concern would need to be addressed in dose-ranging studies where the goal is to normalize the hypertussive state, without severely compromising the protective cough reflex. Studies performed in guinea pigs suggest that NaV1.7, NaV1.8, and NaV1.9 are not only expressed in vagal C-fibers and A-fiber cough receptors, but also expressed in rapidly and slowly adapting low threshold stretch sensitive nerves in the lungs (RARs and SARs) [16]. The effect of inhibiting NaVs in these afferent nerve subtypes may at least potentially lead to unwanted side effects. Little is known about the NaV expression in autonomic nerves in the airways. Inhibition of neural control of vascular tone in the respiratory tract could potentially raise concerns.
References
Catterall WA, Raman IM, Robinson HP, Sejnowski TJ, Paulsen O (2012) The Hodgkin–Huxley heritage: from channels to circuits. J Neurosci 32(41):14064–14073
Catterall WA (2012) Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol 590(Pt 11):2577–2589
Hansson L, Midgren B, Karlsson JA (1994) Effects of inhaled lignocaine and adrenaline on capsaicin-induced cough in humans. Thorax 49(11):1166–1168
Trochtenberg S (1994) Nebulized lidocaine in the treatment of refractory cough. Chest 105(5):1592–1593
Howard P, Cayton RM, Brennan SR, Anderson PB (1977) Lignocaine aerosol and persistent cough. Br J Dis Chest 71(1):19–24
Chong CF, Chen CC, Ma HP, Wu YC, Chen YC, Wang TL (2005) Comparison of lidocaine and bronchodilator inhalation treatments for cough suppression in patients with chronic obstructive pulmonary disease. Emerg Med J 22(6):429–432
Dicpinigaitis PV, Gayle YE, Solomon G, Gilbert RD (2009) Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough. Respir Med 103(6):902–906
Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ (2004) Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557:543–558
Fox AJ, Barnes PJ, Urban L, Dray A (1993) An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. J Physiol 469:21–35
Carr MJ, Kollarik M, Meeker SN, Undem BJ (2003) A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther 304:1275–1279
Chuaychoo B, Lee MG, Kollarik M, Pullmann R Jr, Undem BJ (2006) Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol 575(Pt 2):481–490
Coleridge JCG, Coleridge HM (1984) Afferent vagal C-fiber innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99:1–110
Taylor-Clark T, Undem BJ (2006) Transduction mechanisms in airway sensory nerves. J Appl Physiol 101(3):950–959
Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M (2004) Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556(pt 3):905–917
Duarte AG, Myers AC (2012) Cough reflex in lung transplant recipients. Lung 190(1):23–27
Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ (2008) Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol 586(5):1321–1336
Muroi Y, Ru F, Kollarik M, Canning BJ, Hughes SA, Walsh S, Sigg M, Carr MJ, Undem BJ (2011) Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol 589(Pt 23):5663–5676
Raymond CK, Castle J, Garrett-Engele P, Armour CD, Kan Z, Tsinoremas N, Johnson JM (2004) Expression of alternatively spliced sodium channel alpha-subunit genes. Unique splicing patterns are observed in dorsal root ganglia. J Biol Chem 279(44):46234–46241
Lai J, Porreca F, Hunter JC, Gold MS (2004) Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol 44:371–397
Priest BT, Kaczorowski GJ (2007) Subtype-selective sodium channel blockers promise a new era of pain research. Proc Natl Acad Sci USA 104(20):8205–8206
Nardi A, Damann N, Hertrampf T, Kless A (2013) Advances in targeting voltage-gated sodium channels with small molecules. ChemMedChem 7(10):1712–1740
Farrag KJ, Costa SK, Docherty RJ (2002) Differential sensitivity to tetrodotoxin and lack of effect of prostaglandin E2 on the pharmacology and physiology of propagated action potentials. Br J Pharmacol 135(6):1449–1456
Canning BJ, Mori N (2010) Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 300(2):R369–R377
Mazzone SB, Reynolds SM, Mori N, Kollarik M, Farmer DG, Myers AC, Canning BJ (2009) Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci 29(43):13662–13671
Muroi Y, Ru F, Chou YL, Carr MJ, Undem BJ, Canning BJ (2013) Selective inhibition of vagal afferent nerve pathways regulating cough using NaV1.7 shRNA silencing in guinea pig nodose ganglia. Am J Physiol Regul Integr Comp Physiol 304:R1017–R1023
Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD (2010) ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci 30(5):1637–1647
Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS (2008) Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain 12(5):564–572
Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice M, Fraser R, Young C, Hossain S, Pape T, Payne B, Radomski C, Donaldson G, Ives E, Cox J, Younghusband HB, Green R, Duff A, Boltshauser E, Grinspan GA, Dimon JH, Sibley BG, Andria G, Toscano E, Kerdraon J, Bowsher D, Pimstone SN, Samuels ME, Sherrington R, Hayden MR (2007) Loss-of-function mutations in the NaV1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet 71(4):311–319
Weiss J, Pyrski M, Jacobi E, Bufe B, Willnecker V, Schick B, Zizzari P, Gossage SJ, Greer CA, Leinders-Zufall T, Woods CG, Wood JN, Zufall F (2011) Loss-of-function mutations in sodium channel NaV1.7 cause anosmia. Nature 472(7342):186–190
Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL (1994) A- and C-type rat nodose sensory neurons: model interpretations of dynamic discharge characteristics. J Neurophysiol 71(6):2338–2358
Gold MS (1999) Tetrodotoxin-resistant Na+ currents and inflammatory hyperalgesia. Proc Natl Acad Sci USA 96(14):7645–7649
Kwong K, Lee LY (2002) PGE2 sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol 93(4):1419–1428
Maher SA, Birrell MA, Belvisi MG (2009) Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med 180(10):923–928
Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS (2007) A-803467, a potent and selective NaV1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104(20):8520–8525
Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ (2006) The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci 26(50):12852–12860
O’Neill J, McMahon SB, Undem BJ (2013) Chronic cough and pain: Janus faces in sensory neurobiology? Pulm Pharmacol Ther 26:476–485
Rush AM, Waxman SG (2004) PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res 1023(2):264–271
Ritter AM, Martin WJ, Thorneloe KS (2009) The voltage-gated sodium channel NaV1.9 is required for inflammation-based urinary bladder dysfunction. Neurosci Lett 452(1):28–32
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muroi, Y., Undem, B.J. Targeting Voltage Gated Sodium Channels NaV1.7, NaV1.8, and NaV1.9 for Treatment of Pathological Cough. Lung 192, 15–20 (2014). https://doi.org/10.1007/s00408-013-9533-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9533-x