Abstract
Objectives
Enterobacter cloacae (E. cloacae) is a Gram-negative rod commonly found on intensive care units (ICU) causing severe infections with high mortality. Specific characteristics of E. cloacae pneumonia, however, have not been identified.
Design
Evaluation of clinical and microbiological records of patients with positive respiratory samples for E. cloacae was performed by a 1-year retrospective study in a large university hospital.
Results
Ninety-seven of 115 eligible patients with E. cloacae-positive respiratory samples developed pneumonia. Patients were predominantly male (68%), older (median age = 62 years), and immunodeficient (54%). Seventy-eight percent required ICU admission, of which 97% required mechanical ventilation. Ventilator-associated pneumonia (VAP) occurred in 58%. Those who developed E. cloacae VAP had undergone twice as many surgical procedures under translaryngeal intubation prior to VAP onset (89 vs. 48%, P < 0.0001). Overall, E. cloacae VAP mortality was 24%. In E. cloacae VAP patients, presence of translaryngeal tubes (P = 0.02) and female gender (P = 0.0003) were associated with poor survival. Multivariate analysis confirmed male sex as a protective factor (relative risk: 0.39; P = 0.007).
Conclusion
Enterobacter cloacae causes VAP with high mortality, predominantly in women. Risk factors for E. cloacae pneumonia seem to match those for VAP. The presence of translaryngeal endotracheal tubes seems to be the specific factor for E. cloacae VAP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enterobacter cloacae (E. cloacae) is a commensal from the bacterial genus Enterobacter. It is a peritrichously flagellated, facultative anaerobic, Gram-negative rod-shaped bacterium of the family Enterobacteriaceae [1]. So far, more than 40 species and subspecies of Enterobacter have been identified [2]. Enterobacter sp. are opportunistic pathogens typically found on intensive care units (ICU), with varying incubation times from a few hours up to 20 days [1]. In healthy individuals the gastrointestinal tract represents the most important reservoir [1, 3]. During the last 40 years, the clinical importance of these pathogens has increased, with actual isolation rates of approximately 10% in nosocomial respiratory tract infections [4]. E. cloacae has the highest pathogenic potential corresponding to approximately 60–75% of all Enterobacter infections [1, 3, 5].
Nosocomial lower respiratory tract infections have a high mortality rate but about one third of these infections are considered preventable [6, 7]. Gram-negative bacteria account for about 30% of all hospital-acquired infections and up to 70% of nosocomial infections in the ICU [6]. In addition, nosocomial respiratory tract infections, especially ventilator-associated pneumonia (VAP), from Gram-negative, multidrug-resistant pathogens pose a growing clinical and economic burden [8]. E. cloacae shows constitutive and inducible expression of β-lactamases that generally confer resistance to aminopenicillins and first- and second-generation cephalosporins [9]. Although general risk factors for Enterobacter sp. infections, such as male gender, old age, immunodeficiency, malignant hematological diseases, and intensive care have been identified [1], a systematic characterization of E. cloacae pneumonia in the ICU setting has not been performed thus far.
The aim of the present study was to characterize patients with E. cloacae pneumonia in our center and to evaluate specific aspects of E. cloacae VAP in order to identify possible prevention strategies.
Materials and Methods
Study Design
The study was performed retrospectively from the 1 January 2007 to 31 December 2007. We included eligible patients from all wards of the University Medical Centre Hamburg–Eppendorf (Hamburg, Germany), a 1,489-bed tertiary-care hospital. Included was the Department of Critical Care Medicine comprising three nonsurgical, three surgical, and one interdisciplinary unit, totaling 84 ICU beds at the time of the study. The institutional data safety officer waived the need for obtaining written consent from the patients as this was a retrospective, noninterventional study.
A VAP prevention protocol had been initiated at our hospital prior to the study. It included the use of an elevated head position, regular cuff suction, and specialized nursing measures. Regular selective digestive tract decontamination was implemented as clinical routine in 2007.
All cases with positive cultures for E. cloacae in respiratory specimens (see below) were identified using the database of the Department of Medical Microbiology, Virology and Hygiene. Patients’ charts were retrieved and relevant data concerning demographics, radiological findings, chemistry, suspected and final diagnosis, comorbidities, immunosuppression, treatment, and outcome were extracted. Patients younger than 18 years or without sufficient clinical information regarding type of pneumonia, comorbidities, and demographic, serological, microbiological, or radiological data were excluded from the study.
Cases were categorized as nonpathogenic colonization of the respiratory tract, community-acquired pneumonia (CAP), nosocomial/hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP). CAP was defined as pneumonia occurring within 48 h after hospitalization without prior residence in a health-care institution such as a nursing home. HAP was defined as pneumonia with onset more than 48 h after hospital admission. VAP was defined as HAP occurring at least 48 h after endotracheal intubation. Patients were classified as apathogenically colonized when E. cloacae was isolated from respiratory samples without signs or symptoms of pneumonia.
Diagnostic criteria for pneumonia were defined according to accepted standards [10]: new or persistent pulmonary infiltrates, consolidations, or pleural effusion on chest radiographs; purulent bronchopulmonary secretions or sputum; clinical signs of pneumonia such as fever, cough, and rales on auscultation of the lung; and raised serum levels of C-reactive protein, procalcitonin, and/or interleukin-6 in combination with isolation of E. cloacae in respiratory materials.
Immunosuppression was defined as the presence of any cause of immunodeficiency, including AIDS, radiation therapy, systemic chemotherapy, post organ transplantation, or glucocorticoid therapy with a daily dose of >10 mg prednisolone or equivalent.
Statistical Analysis
Previously described general risk factors for Enterobacter sp. infections were evaluated [1]: male sex, age >65 years, immunodeficiency, intensive care, presence of malignancy, substance abuse, and indwelling catheters. Univariate statistical analyses were performed using JMP 5.0.1 software (SAS Institute Inc., Cary, NC, USA) for Pearson’s χ2 test, Student’s t-test, Mann–Whitney-test, or ANOVA where appropriate.
Univariate survival analyses were carried out using the Kaplan–Meier method. Multivariate analysis was performed utilizing the Cox regression model. P values of less than 0.05 were regarded as statistically significant.
VAP rate per 1,000 ventilator days was calculated as follows: The total number of all VAP episodes in all ICUs of the center per year were divided by 1,000 times the total number of ventilator days in all ICUs of the center per year.
Results
Study Population
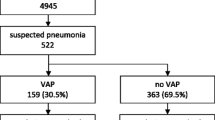
During the study period E. cloacae was detected in respiratory tract specimens from 137 patients. Pediatric patients (n = 13) and cases with incomplete documentation (n = 9) were excluded, leaving 115 cases that formed the study population. Of the 115 patients, 90 were treated on the ICU (78%) and 87 of these 90 patients (97%) required mechanical ventilation (MV). The median time of these patients on MV was 20 days (range = 0–90 days).
The study population was predominantly male (68%) and older (mean age = 62 years, range = 18–87). The most frequent underlying diseases were neurological (38%), bronchopulmonary (34%), and cancer (32%). About half of the patients were immunodeficient. Overall mortality in the study population was 20% (23 of 115 patients). Nineteen patients died in the ICU (21% of ICU patient subgroup equaling 83% of all fatalities). While only 15% of the male patients died in hospital (12/78), the mortality rate within the female study population was twice as high (30%, 11/37, P = 0.07). Details of the characteristics of the study population can be found in Table 1.
Most patients included in this analysis had E. cloacae-associated VAP (58%), followed by CAP (17%), asymptomatic colonization (16%), or HAP (10%) (Table 2).
Pneumonia subgroups differed significantly by age: individuals with E. cloacae CAP were the youngest (median age = 57 years), whereas patients with E. cloacae HAP were the oldest (median age = 70 years, P < 0.05). Mortality was lowest in CAP (5%) and highest in VAP (24%) but this difference did not reach statistical significance (P = 0.35).
Microbiological Analysis (Sampling, Coisolates, Isolation Sites, Resistance, and Colonization)
Respiratory tract specimens were taken from all cases of suspected or clinically apparent pneumonia. Samples were gathered by endobronchial aspiration (74% of all cases included in the study), bronchoalveolar lavage (19%), or sputum (7%) and processed according to standard clinical routines. There was no apparent E. cloacae outbreak detectable.
The most common coisolates with E. cloacae were Candida species (25% of all, mostly C. albicans [23 of 29]), followed by coagulase-negative staphylococci (21%), Staphylococcus aureus (20%), alpha-hemolytic streptococci (17%), Klebsiella spec. (11%), Stenotrophomonas sp. (8%) and Escherichia coli (6%). E. cloacae alone was isolated in 15% of the respiratory samples.
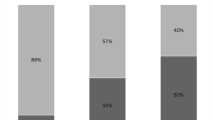
The presence of coisolated microorganisms varied between VAP and non-VAP patients: whereas Staphylococcus aureus was present in VAP almost three times as often as in non-VAP cases (27 vs. 10%, P < 0.05), coagulase-negative staphylococci occurred three times as often in samples from non-VAP patients (9 vs. 27%, P < 0.01). Yet, in about every fifth case, E. cloacae alone was the cause of VAP (21 vs. 6%, P < 0.05, Fig. 1).
In vitro, all but one of the E. cloacae isolates were resistant to aminopenicillins. VAP patients had an increased rate of ureidopenicillin-resistant E. cloacae strains compared with non-VAP patients (28 vs. 10%, P < 0.05). However, microbial resistance rates against standard therapeutics (e.g., carbapenems, aminoglycosides, and fluoroquinolones) were clinically insignificant.
Oropharyngeal samples were taken from 57 patients (50% of the total) and 19 of these were colonized with E. cloacae (33%). No differences between patients with VAP and patients without VAP were observed. However, ICU patients treated in nonsurgical units had a significantly higher rate of colonization than patients on surgical ICUs (9/15, 60% vs. 8/36, 22%, P < 0.01). Patients with neurological disorders showed a greater proportion of colonization (12/24, 50%, P < 0.05), while patients with underlying bronchopulmonary diseases were less frequently colonized oropharyngeally (12%, P < 0.05).
ICU Versus Normal Care
On normal-care (NC) wards bronchopulmonary and oncological disorders were the most common underlying diseases (62% and 58%, respectively, P < 0.01 each; Table 1). Lung cancer was the most frequent malignancy on NC wards (60%) followed by hematological malignancies (27%). In contrast, cancer of the upper digestive tract represented the most common malignancy within the ICU group (45% of all cancers).
Previously described risk factors for Enterobacter include alcohol abuse, smoking, and immunodeficiency [3].While high alcohol intake was rare among patients of both groups (16 vs. 11%), many were smokers (46 vs. 64%). Approximately half of all patients on ICU and NC wards were immunosuppressed. The same rate of patients on ICU and NC wards showed asymptomatic colonization (40 vs. 39%). A subgroup analysis of immunodeficient patients revealed a trend toward increased oropharyngeal colonization in this group of patients but this did not reach statistical significance (43 vs. 22%, P = 0.09).
Most interestingly, the majority of E. cloacae-positive individuals on ICU (89%) had undergone surgical procedures under general anesthesia prior to isolation of the pathogen compared to only 20% of the patients on regular wards (P < 0.0001, more details in Table 1).
Apathogenic Deep Respiratory Tract Colonization with E. cloacae
Of the 18 patients colonized with E. cloacae, 76% featured therapy- or illness-related immunodeficiency (P < 0.05). These patients received mostly glucocorticoids (5 of 18, 28%) or systemic chemotherapy (5/18, 28%). Nine patients (50%) suffered from malignoma, predominantly squamous cell carcinoma of different origins (3/18, 17%). Sixty-one percent of the patients (11/18) were male and 10 were older than 65 years (56%). Ten of these patients had been treated on surgical wards (10/18, 56%). Most colonized patients were referred to ICU or intermediate-care units (IMC) before isolation of E. cloacae (12/18, 67%). Ten patients had indwelling central venous catheters (56%) at the time of diagnosis. Only two patients had a documented substance abuse (smoking); no patient apathogenically colonized suffered from alcoholism (see Table 2 for further information). Neither immunosuppression nor type of immunosuppression influenced survival of the patients colonized with E. cloacae (both P > 0.05). For information on upper airway tract and oropharyngeal colonization, see Tables 1 and 3.
Enterobacter cloacae CAP
In total, 12 out of 19 CAP cases were secondary to aspiration (63%), mostly due to underlying neurological disorders (7/12, 58%). Immunosuppression was present in ten CAP patients (56%). These CAP patients were on steroid medication (5/19, 26%), on systemic chemotherapy (2/19, 11%), had AIDS (2/19, 11%), or had radiation therapy (1/19, 5%). Neither immunosuppression nor type of immunosuppression influenced survival of patients suffering from E. cloacae CAP (both P > 0.05). Ten patients with E. cloacae CAP (10/19, 53%) were instrumentalized with central lines; in nine of these patients catheters were placed within 2 days before isolation of the pathogen.
Because it has been described that E. cloacae CAP presents as necrotizing pneumonia [11, 12], we investigated whether there was a typical pattern on chest X-ray examinations in our patients. A typical radiographic pattern was not identifiable and cavitational pulmonary lesions were present in only 16% of our CAP patients (3/19).
E. cloacae VAP
About two thirds of all patients with E. cloacae pneumonia had VAP, which occurred mostly as late VAP, with onset more than 5 days after endotracheal intubation (42/60, 70%). Patients with late E. cloacae VAP were 10 years younger on average (mean age ± SEM: 66 ± 3.7 vs. 56 ± 2.5 years, P < 0.05) and were less likely to have digestive tract (50 vs. 19%, P < 0.05) or oncological disorders (50 vs. 17%, P < 0.01) than those with early VAP. We also observed a trend toward an increased prevalence of severe neurological (22 vs. 48%, P = 0.07) or bronchopulmonary diseases (17 vs. 38%, P = 0.10) in those with late VAP. Systemic inflammatory response and sepsis seemed to occur more often in patients with early E. cloacae VAP (61 vs. 38%, P = 0.09). We observed an E. cloacae VAP rate of 37.1 cases per 1,000 ventilator days. In other words, 67 of 87 (77%) mechanically ventilated patients with E. cloacae present in respiratory fluids developed VAP. Although there was no total difference in immunosuppressed individuals between both groups, VAP patients have more often been treated with glucocorticoids (>10 mg prednisolone or equivalent) than patients suffering from other E. cloacae pneumonia (39 vs. 22%). On the other side, non-VAP patients more often had radiation or chemotherapy (5 vs. 14%, P< 0.05).
In the VAP patients, women had a significantly higher overall mortality rate than men (43 vs. 15%, P < 0.05); however, fewer women suffered from E. cloacae VAP overall (31 vs. 69%).
There was no detectable difference in oropharyngeal colonization rates between VAP and non-VAP groups, but patients with E. cloacae VAP had twice as many surgical procedures with translaryngeal endotracheal intubation performed prior to onset of pneumonia corresponding to 61 of 67 patients (91 vs. 48%, P < 0.0001, see Table 3 for full details).
Possible Risk Factors for E. cloacae VAP Mortality
In the Kaplan–Meier analyses, female sex and the presence of translaryngeal endotracheal tubes were associated with an unfavorable outcome for E. cloacae VAP (Fig. 2a, b, P = 0.0003 and P = 0.02, respectively). However, a trend toward poor survival was also detected for immunocompromised patients (Fig. 2e, P = 0.11), those without underlying hematological malignancies (Fig. 2d, P = 0.11), and those treated on medical ICUs (Fig. 2c, P = 0.08). No other tested conditions such as age (Fig. 2f, P = 0.33), noncancerous comorbidities, substance abuse, isolation site, coisolates, or onset of VAP revealed any significant associations with prognosis. Additionally, the type of immunosuppression did not influence survival (P = 0.71).
Survival of patients with E. cloacae VAP. Kaplan–Meier analyses of 67 patients suffering from E. cloacae VAP. Survival is gender-dependent (a, P = 0.0003) and impaired in patients with prolonged translaryngeal endotracheal intubation (b, P = 0.02). Trends toward poorer prognosis can be detected in immunodeficient (d) patients treated on medical ICUs (c) and not suffering from oncological disorders (e). Age, an independent risk factor for general VAP outcome, does not affect E. cloacae VAP survival (f). Median follow-up after isolation of E. cloacae from respiratory samples was 20 days (range = 0–92 days)
A multivariate analysis that included sex, age, airway management, presence of malignant disease, type of intensive care, and immunological status identified only female sex (P = 0.0066) as an independent predictor of poor outcome. However, an unfavorable trend toward the presence of translaryngeal endotracheal tubes was observed (P = 0.09, see Table 4).
Discussion
VAP is a potentially lethal disease and a costly burden within intensive care medicine [8, 13]. Gram-negative bacilli are involved in 60–70% of all VAP cases [14]. Enterobacter are among the most common Gram-negative bacteria isolated from the respiratory tracts of VAP patients after inadequate antibiotic pretreatment [8]. E. cloacae is the most virulent of the Enterobacter species [1].
In our study population, E. cloacae was present mainly in respiratory tract samples of patients in the ICU (78% of all patients in this study). Virulence seems to be high: only 16% of all patients were apathogenically colonized. This finding is supported by the particularly high E. cloacae VAP rate of 37.1 per 1,000 ventilator days within our study population, which is about ten times higher than the actual VAP rate of 3.89 per 1,000 ventilator days within the complete Department of Critical Care Medicine in 2008. However, this could be attributed to the selection bias in the microbiological sampling as most of the samples were taken in the ICU or the bronchoscopy unit due to the presence of respiratory tract infections. This most likely leads to overselection of symptomatic patients who are infected with E. cloacae. The true colonization rate or E. cloacae prevalence therefore cannot be identified in this retrospective study.
E. cloacae pneumonia, and especially E. cloacae VAP, generally can be regarded as a polymicrobial infection with a high coincidence of Candida sp., Staphylococcus aureus, and Gram-negative bacteria; however, every fifth case of E. cloacae VAP within our study was exclusively caused by this pathogen. If coisolation of Candida albicans is regarded as colonization rather than infection of the lung [15], the rate of VAP caused solely by E. cloacae increases to 40% in our patients. We found that the presence of additional pathogens did not affect the mortality of E. cloacae VAP patients.
Possible Risk Factors for Acquisition and Poor Outcome of E. cloacae VAP
General risk factors previously identified for other Enterobacter sp. infections and bacteremia also seem to be applicable to E. cloacae pneumonia. Patients at risk in our study were mostly older men who were being treated on ICU with severe underlying pathologies such as neurological, bronchopulmonary, or oncological disorders. Surprisingly, abusive alcohol intake was infrequent within our study population (15% of all), but this may be attributed to incomplete documentation. Smoking, in contrast, was frequent. Central venous catheters were present in 62% of the ICU patients.
Intensive care in general has been identified as a risk factor for Enterobacter (bloodstream) infections [1]. This also seems to hold true for E. cloacae pneumonia, since 66% of patients with HAP or apathogenic colonization within our study had been treated on the ICU before isolation of E. cloacae. Most of the above-mentioned factors have also been characterized as general risk factors for VAP [13].
VAP caused by E. cloacae is a severe and life-threatening condition: in our study 16 of 19 patients who died in the ICU died from E. cloacae VAP (84%). Nevertheless, all-cause mortality in our sample is within the range of overall VAP mortality [14]. Although more men had E. cloacae VAP, the mortality rate in women was higher in our study population. Female gender had previously been shown to be a general risk factor for poor VAP outcome [16]. The same holds true for E. cloacae VAP. We identified female sex as an independent unfavorable factor for death from E. cloacae VAP. Surprisingly, all other tested conditions, including immunosuppression, presence of hematological malignancies, and age, were not significantly associated with a poor outcome in univariate or multivariate analysis.
Endotracheal Intubation as Principal Factor?
From our study, endotracheal intubation appears to be the most important factor for acquiring E. cloacae pneumonia. Similar proportions of patients on normal-care and intensive care units, respectively, had oropharyngeal colonization with E. cloacae (36 vs. 40). Most patients with upper-airway colonization were also immunosuppressed (76%). The striking difference between patients colonized and those developing E. cloacae pneumonia seems to be the surgical procedure under general anesthesia with translaryngeal endotracheal intubation before the onset of E. cloacae pneumonia/VAP. Although the proportion of upper-airway colonization differed significantly between nonsurgical and surgical ICUs, with a lower incidence on surgical wards, the vast majority of E. cloacae VAP occurred on surgical ICUs (77% of all). Additionally, we observed a trend toward a higher risk of dying from E. cloacae VAP in patients with translaryngeal endotracheal tubes rather than dilatational or surgical tracheotomies according to the Kaplan–Meier analysis.
Taken together, we believe that translaryngeal endotracheal intubation is the principal risk factor for acquiring and maintaining E. cloacae pneumonia: Translaryngeal intubation may move E. cloacae from the upper to the lower respiratory tract and maintain it there. The presence of E. cloacae in the upper respiratory tract alone appears not to be sufficient to cause E. cloacae pneumonia in most cases, even in the presence of immunosuppression. This becomes obvious in light of the high rate of apathogenic E. cloacae colonization in immunosuppressed patients.
Therefore, special protective measures should be considered, including strict personnel hygiene, selective (digestive tract or oropharyngeal) decontamination (SDD or SOD [17]), and the use of specially coated endotracheal tubes [18]. In other centers, Enterobacter and E. cloacae outbreaks have been traced to poor personnel hygiene and contaminated devices such as stethoscopes, endoscopes, invasive monitoring devices, and even intravenous solutions or cotton swabs [1, 19]. Consequently, the highest hygiene standards should be applied on ICU.
Our findings need to be confirmed by further investigations due to the intrinsic limitations of a retrospective cohort study lacking the power of a controlled, matched, or randomized trial.
Conclusions
E. cloacae is a pathogen with high virulence predominantly causing VAP with high mortality. Women have a higher risk of dying from E. cloacae VAP. The risk factors for E. cloacae pneumonia seem to match the risk factors for VAP. A specific factor for E. cloacae VAP, however, seems to be the presence of translaryngeal endotracheal tubes.
References
Sanders WE, Sanders CC (1997) Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev 10:220–241
WFCC (2011) World federation for culture collections, WDCM databases. http://www.wfcc.info/wdcmdb/
Lin YC, Chen TL, Ju HL, Chen HS, Wang FD, Yu KW, Liu CY (2006) Clinical characteristics and risk factors for attributable mortality in Enterobacter cloacae bacteremia. J Microbiol Immunol Infect 39:67–72
Gastmeier P, Sohr D, Geffers C, Rüden H, Vonberg RP, Welte T (2009) Early- and late-onset pneumonia: is this still a useful classification? Antimicrob Agents Chemother 53:2714–2718
Al-Tawfiq JA, Antony A, Abed MS (2009) Antimicrobial resistance rates of Enterobacter spp.: a seven-year surveillance study. Med Princ Pract 18:100–104
Peleg AY, Hooper DC (2010) Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med 362:1804–1813
Yokoe DS, Mermel LA, Anderson DJ, Arias KM, Burstin H, Calfee DP, Coffin SE, Dubberke ER, Fraser V, Gerding DN, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Marschall J, Nicolle L, Pegues DA, Perl TM, Podgorny K, Saint S, Salgado CD, Weinstein RA, Wise R, Classen D (2008) A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol 29(Suppl 1):S12–S21
Amin A (2009) Clinical and economic consequences of ventilator-associated pneumonia. Clin Infect Dis 49(Suppl 1):S36–S43
Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV (2007) Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol 45:3352–3359
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Musher DM, Niederman MS, Torres A, Whitney CG (2007) Infectious diseases society of America/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72
Karnad A, Alvarez S, Berk SL (1987) Enterobacter pneumonia. South Med J 80:601–604
Broughton WA, Kirkpatrick MB (1988) Acute necrotizing pneumonia caused by Enterobacter cloacae. South Med J 81:1061–1062
Kollef MH (2004) Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med 32:1396–1405
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Kontoyiannis DP, Reddy BT, Torres HA, Luna M, Lewis RE, Tarrand J, Bodey GP, Raad II (2002) Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis 34:400–403
Combes A, Luyt CE, Fagon JY, Wolff M, Trouillet JL, Chastre J (2007) Early predictors for infection recurrence and death in patients with ventilator-associated pneumonia. Crit Care Med 35:146–154
de Smet AM, Hopmans TE, Minderhoud AL, Blok HE, Gossink-Franssen A, Bernards AT, Bonten MJ (2009) Decontamination of the digestive tract and oropharynx: hospital acquired infections after discharge from the intensive care unit. Intensive Care Med 35:1609–1613
Pacheco-Fowler V, Gaonkar T, Wyer PC, Modak S (2004) Antiseptic impregnated endotracheal tubes for the prevention of bacterial colonization. J Hosp Infect 57:170–174
Kanemitsu K, Endo S, Oda K, Saito K, Kunishima H, Hatta M, Inden K, Kaku M (2007) An increased incidence of Enterobacter cloacae in a cardiovascular ward. J Hosp Infect 66:130–134
Acknowledgments
Presented in part as an oral presentation at the 41st joint meeting of the German Society for Intensive Care and Emergency Medicine and the Austrian Society for Intensive Care Medicine by JKH. JKH received a postdoctoral research fellowship from the “Hubertus Wald Tumorzentrum—University Cancer Centre Hamburg” during part of this study.
Disclosure
Drs. Hennigs, Baumann, Schmiedel, Tennstedt, Sobottka, Bokemeyer, Kluge, and Klose do not have any personal or financial support or involvement with organization(s) that have financial interest in the subject matter or any conflicts of interest to report for the purpose of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. K. Hennigs and H. J. Baumann contributed equally to this study.
Rights and permissions
About this article
Cite this article
Hennigs, J.K., Baumann, H.J., Schmiedel, S. et al. Characterization of Enterobacter cloacae Pneumonia: A Single-Center Retrospective Analysis. Lung 189, 475–483 (2011). https://doi.org/10.1007/s00408-011-9323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-011-9323-2