Abstract
Cell deformability of all major blood cell types is increased in depressive disorders (DD). Furthermore, impaired glucocorticoid secretion is associated with DD, as well as depressive symptoms in general and known to alter cell mechanical properties. Nevertheless, there are no longitudinal studies examining accumulated glucocorticoid output and depressive symptoms regarding cell deformability. The aim of the present study was to investigate, whether depressive symptoms predict cell deformability one year later and whether accumulated hair glucocorticoids mediate this relationship. In 136 individuals (nfemale = 100; Mage = 46.72, SD = 11.28; age range = 20–65), depressive symptoms (PHQ-9) and hair glucocorticoids (cortisol and cortisone) were measured at time point one (T1), while one year later (T2) both depressive symptoms and hair glucocorticoids were reassessed. Additionally, cell deformability of peripheral blood cells was assessed at T2. Depression severity at T1 predicted higher cell deformability in monocytes and lymphocytes at T2. Accumulated hair cortisol and cortisone concentrations from T1 and T2 were not associated with higher cell deformability and further did not mediate the relationship between depressive symptoms and cell deformability. Elevated depressive symptomatology in a population based sample is longitudinally associated with higher immune cell deformability, while long-term integrated glucocorticoid levels seem not to be implicated in the underlying mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of all mental disorders, depressive disorders (DD), comprising major depressive disorder (MDD) and persistent depressive disorder (PDD), cause the greatest disease burden and disability worldwide [1], being top-ranked among an exhaustive number of fatal and non-fatal diseases causing disability-adjusted life-years in all ages [2]. MDD is, according to the classification system DSM-5 [3] characterized by a period of at least two weeks of depressive mood or anhedonia in combination with other symptoms such as appetite changes, insomnia/hypersomnia, increased fatigue, or feelings of worthlessness. PDD (formerly dysthymia) is characterized on the basis of depressed mood over a two-year period in combination with symptoms similar to MDD, although PDD is often perceived as less severe in terms of intensity [3]. Nevertheless, both conditions cause severe functional impairment and there are major difficulties in reducing the high prevalence rates [4, 5] and improving DD treatment [6, 7], with up to 30% of patients not achieving remission despite multiple treatment attempts [8,9,10]. Often, the poor therapeutic efficacy is attributed to the still insufficiently understood pathophysiology of DD [11,12,13,14].
There is accumulating evidence of key pathophysiological processes in depression and its etiology occurring directly at the cellular level and that DD and depressive symptoms can impair peripheral blood cell function via disturbed glucocorticoid secretion by the hypothalamus-pituitary-adrenal (HPA) axis or via inflammation [15,16,17,18,19,20,21]. Blood cells fulfill multiple functions from primary immune response, over metabolite transport to overall blood flow. Real-time deformability cytometry (RT-DC) represents a new method which enables the investigation of physical properties of blood cells (e.g., cell mechanical features, cell size), thereby providing unprecedented insights into overall blood cell function [22, 23]. Previous research indicates that the assessment of the mechanical state of blood cells, measured by cell deformability, is appropriate to detect and classify human diseases conditions such as spherocytosis, malaria, or COVID-19 infections [24, 25] and is predictive for immune cell activation [26, 27].
Only recently, using RT-DC, we were able to show that in DD and especially in PDD immune cell deformability is increased as compared to healthy controls [28]. Although all major immune cells tended to be more deformable, lymphocytes, monocytes and neutrophils were particularly affected indicating immune cell mechanical changes to occur in DD. This is potentially related to disturbed HPA axis function or dysfunctional immune response, both significantly grounded as prominent biological findings in MDD [18] affecting the cell architecture [29, 30]. In addition, optical traps [31], atomic force microscopy [23, 32], and micropipette aspiration [33] have been used in proof-of-concept studies to reveal mechanical changes in immune cells under pathological conditions. Nevertheless, due to the general dominance of erythrocytes in blood and the consequently increased statistical power to detect associations with cell morphological or mechanical changes, previous studies of interest investigated only the relationship between erythrocyte deformability and a developmental disorder as well as a multi-systemic disease with fatigue as leading symptom [34, 35]. The study by Jasenovec et al. (2019) examined children within the autism spectrum identifying those children with more severe symptoms to show impaired erythrocyte deformability. Further, Saha et al. (2019) reported patients with chronic fatigue syndrome / myalgic encephalomyelitis to exhibit lower erythrocyte deformability than healthy controls. It should be noted here, however, that the current gold standard for measuring cell deformability, namely RT-DC [22, 36,37,38,39], was only used in our landmark study comparing individuals with DD and healthy controls [28].
There is a large body of literature examining the relationship between impaired glucocorticoid secretion by the HPA axis, DD [14] and depressive symptoms [15]. Indeed, for some time, it seemed to be widely accepted that MDD and depression severity are associated with increased HPA activity and increased basal cortisol concentrations in the circulation [13, 40]. However, due to the inconclusive findings on cortisol stress reactivity in MDD, this is no longer definitive [14, 41, 42]. Furthermore, investigating new tissue sources such as hair showing mixed associations with regard to DD highlight on the one hand the relevance of the investigation of chronic glucocorticoid secretion over longer time periods [14, 43,44,45,46,47], but on the other hand, it emerges as increasingly important to investigate the effects of impaired chronic glucocorticoid secretion on cell function. Studies increasingly show that the extracellular microenvironment is critical to modify cellular physiology, which ultimately determines cell functionality [48]. Therefore, understanding the interactions between immune cells and DD-related alterations in glucocorticoid secretion appears to be increasingly important for understanding differences in immune cell deformability in health and disease.
In addition to increased cortisol levels and the often identified chronic low-grade inflammation in individuals suffering from DD [18], Lynall et al. (2020) further reported elevated numbers of immune cells in screened depressed individuals compared to controls [49]. It is known that increased levels of glucocorticoids and catecholamines lead to increased immune cell count, as cells demarginate from the vessel walls. Interestingly, these observations were associated with cellular softening [29]. It is suggested that the underlying mechanism leading to increased immune cell counts in depressed individuals is rooted in the effect of elevated glucocorticoid levels remodeling the actin cytoskeleton of blood cells and thereby softening leukocytes and enabling them to demarginate from the vessel wall [29]. Not only starts the actin cytoskeleton to be remodeled with continuously elevated levels of glucocorticoids, but also are lipid metabolism and composition crucially affected resulting in increased softening and bending of blood cells [20, 33, 50,51,52]. This cascade of processes ultimately impairs blood cell function and may be an underlying cause of symptoms of fatigue and exhaustion in DD. The disturbed immune cell function in connection with subsequent dysregulated cytokine release could trigger sickness behavior with anhedonia and fatigue [53].
Therefore, we hypothesize that high levels of depressive symptoms are longitudinally associated with increased immune cell deformability. We further hypothesize that continuously high glucocorticoid levels over one year are associated with elevated immune cell deformability and mediate the relationship between depressive symptoms and immune cell deformability.
Method
Study design
This observational study used a longitudinal design with a baseline measurement and a one-year follow-up measurement. Cross-sectional results of the follow-up study are reported elsewhere [28]. Baseline examination of participants took place between October and December 2018, while follow-up examinations were conducted approximately one year later. The follow-up study, entitled Mood-related Morpho-rheological Changes in Peripheral Blood Cells (Mood-Morph), used a participant pool of a large-scale prospective cohort study to identify eligible participants and obtain pre-assessed baseline data (study details of the cohort study are provided elsewhere [54]). Eligible participants were asked via e-mail whether they were willing to participate in an add-on study to the prospective longitudinal study.
For the study, participants with elevated depressive symptoms, as measured by the German version of the Patient Health Questionnaire (PHQ-9 [55]), and gender- and age-matched healthy controls were identified from the participant pool. These participants provided for the baseline measurement self-report data on (T1) sociodemographic information, depressive symptomatology and further health-related factors. Also, collected hair samples for glucocorticoid quantification were obtained at T1. At the follow-up time point (T2), the psychometric assessment and hair sampling was repeated. In addition, blood samples were obtained to measure cell deformability. The study was approved by the local ethics committee of the Dresden University of Technology (EK182042019) and all participants gave written informed consent to participate in the study. For study expenses, participants received cash or a cinema voucher worth 15 € in T1 and 15 € in T2.
Participants
Study inclusion was a participants’ age between 18 and 68 years and sufficient German language skills. Furthermore, in terms of depression levels measured with the PHQ-9, only subjects with a T1 PHQ-9 score > 10 (high risk of depression) as well as age- and sex-matched subjects with a T1 PHQ-9 score < 5 (low risk of depression) were included. The aim of this procedure was to obtain a sample of participants with high and low depressive symptoms at baseline and to be able to identify change in depressive symptoms over one year. Interested study participants were excluded if they had any kind of blood disorder that could affect blood cell deformability. Potential participants who were contacted to participate in the Mood-Morph study (the detailed sample recruitment can be taken from Walther et al. (2022)), were informed in the study invitation and informational email that certain diseases, such as spherocytosis and blood-related diseases in general, precluded participation [25]. Potential participants with a cold or other acute infections no longer than two weeks ago were not included to exclude acutely infectious participants. Acute medical conditions such as blood diseases, cancer, severe cardiovascular diseases, or pregnancy led to exclusion, while in the population very prevalent conditions such as hypertension or hypothyroidism did not lead to exclusion. These conditions were recorded and used as covariate in the analyses in form of drug specific categories (e.g., for hypertension—antihypertensive drugs).
Procedures
Participants provided sociodemographic and health-related information (e.g., gender, age, medical conditions, medication) as well as depressive symptom severity and information on their general health online via the study-homepage platform. By using a personalized code, participants accessed a private study space, signed first consent forms regarding data privacy, clinical data collection and saving, and hair/blood sampling and completed subsequently study questionnaires. For depression severity the PHQ-9 [55] and for general health the Short Form Health Questionnaire (SF-12 [56]) was completed. While the SF-12 measures general physical and psychological health, the PHQ-9 is a nine-item self-report questionnaire for the measurement of depression severity, rating the frequency of DSM-IV / DSM-5 diagnostic criteria for MDD (e.g., feeling down, depressed, or hopeless) during the past two weeks on a four-point Likert scale (0 = not at all, 3 = nearly every day). Scores ≥ 10 were consistently shown to identify with high sensitivity and specificity cases of MDD [57, 58].
To obtain capillary blood and scalp hair samples at T2, participants arrived at the Dresden University of Technology at the Department of Biopsychology, where trained personnel performed blood and hair sampling. After the bio-sampling took place, the depressive episode section of a standardized interview, named the Composite International Diagnostic Interview (DIA-X-5/CIDI) was conducted by trained interviewers [59]. To rule out bipolar disorders, a mania-screening questionnaire, consisting of the initial questions of the DIA-X-5 (hypo-)manic episodes’ section, was conducted to detect any lifetime (hypo-) mania-symptoms. Analyses regarding differences in cell deformability between DD (MDD and PDD) and healthy participants can be taken from Walther et al. (2022) and are not part of this manuscript. Duration of the examination at T2 was approximately one hour.
Real-time deformability cytometry (RT-DC)
A 20 µl capillary blood sample was extracted from the fingertip of participants using a safety lancet (Safety-Lancet Normal 21, Sarstedt, Nümbrecht, Germany) and harvested in a capillary (Minivette POCT, 20 µl, Sarstedt, Nümbrecht, Germany). The blood was immediately resuspended in 380 µl RT-DC measurement buffer containing 0.6% methylcellulose in phosphate buffered saline (CellCarrierB, Zellmechanik Dresden, Germany) in a microcentrifuge tube. Blood-samples were then transferred to the Department of Cellular Machines at the Biotechnology Center of the Dresden University of Technology, where the samples were measured using an RT-DC device. Maintained at room temperature, samples were measured within three hours since sampling according to a protocol published elsewhere [25].
In brief, blood was flushed through a microfluidic channel constriction 20 μm x 20 μm in cross section (Flic20, Zellmechanik Dresden, Germany) by applying a constant flow rate. An image of every measured blood cell was taken by a high-speed camera and cell deformability and cell size were calculated [22]. RT-DC measurements were controlled by the acquisition software Shape-In2 (Zellmechanik Dresden, Germany). The different blood cell types were classified by utilizing artificial intelligence-based image classification as published elsewhere [36] and mean values for cell deformability and cell size of every donor and blood cell type were extracted. Subsequently, we only focus on immune cells and erythrocytes.
Hair sampling and liquid chromatography and mass-spectrometry (LC-MS)
Three hair strands (each containing at least 20 mg of hair) were cut as close as possible to the scalp from the posterior vertex for quantification of hair cortisol and cortisone. Hair sample processing and analysis were performed as described in our published protocols for glucocorticoids [60, 61]. Preprocessing of the samples and subsequent biochemical analysis were performed by Dresden LabService GmbH (Tatzberg 47, 01307, Dresden, Germany). Samples with a minimum hair length of 3 cm were cut into 3 cm segments from the scalp site representing the integrated hormone concentration over the last three months (e.g., average hair growth rate of 1 cm per month [62]) and weighed into 7.5 mg of whole, non-pulverized hair and then washed with 2.5 ml of isopropanol according to the protocol of Gao et al. (2013). Biochemical analysis was performed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) as described in detail elsewhere [61]. The intra- and inter-assay coefficients of variation for cortisol and cortisone are less than 8.8% and 8.5%, respectively. The lower limit of detection for cortisol and cortisone is 0.09 pg/mg.
Statistical analysis
Statistical analyses were run in R v4.3.0 (RStudio v. 2023.06.0 [63]). The longitudinal association between depressive symptoms at T1 and cell deformability at T2 (lymphocytes, monocytes, neutrophils, granulo-monocytes [cluster of monocytes and all cell types of granulocytes] and erythrocytes) was examined by hierarchical regression analyses with bootstrapping (R = 1000) and robust standard errors (HC4) to obtain robust effect estimations for the entire sample (n = 136). Data was checked for linearity, normal distribution of the residuals, heteroscedasticity, multicollinearity and outliers. Hierarchical regression analyses were conducted in two steps: First, age, BMI, gender, and psychopharmaceutical intake, measured at T2, were included as confounders. Second, with respect to the analyses for the entire sample, depressive symptoms at T1 were included in the regression model to account for additionally explained variance. Additionally, we run a mediation analyses with the process macro, provided by Hayes (2022) [64], to examine the mediating effect of accumulated hair glucocorticoids (cortisol and cortisone at T1 + T2, respectively) between depressive symptoms (PHQ-9 at T1) and cell deformability at T2. P-values were adjusted using Holm-Bonferroni correction for multiple comparisons [65] by the number of cell types. Cortisol and cortisone data from both measurements were log-transformed due to their non-normal distribution. All hypotheses were tested two-tailed in regard to cell deformability. Pearson and Kendall correlation coefficients [66] were tested to check for possible (non-)linearity relationships between depressive symptom severity and cell area as well as depressive symptom severity or glucocorticoids and cell count, obtained by the classification algorithm [36]. ANOVA was used to check for any differences in medication intake groups and cell deformability for each cell type.
Results
Sample description
In total, 136 (nfemale = 100) individuals, who completed the PHQ-9 at T1 and T2 were used for subsequent analyses investigating the longitudinal association between depressive symptoms, glucocorticoid secretion and immune cell deformability. Sample characteristics of the entire sample are presented in Table 1. Moreover, hair segments could not be obtained from all subjects due to lack of sufficient hair of some participants. Regarding cortisol analyses, one outlier was additionally excluded due to a hair cortisol concentration above 100 pg/mg. Regarding cortisol output n = 113 observations were available at both time points, while for cortisone output n = 115 observations were available at both time points.
Control of confounders
No significant correlations between depressive symptoms (PHQ-9 T1) and mean cell area size (see Supplementary Table 1) and between depressive symptoms (PHQ-9 T1) and cell count (see Supplementary Table 2) were found for any cell type. Furthermore, accumulated hair cortisol and cortisone levels were not associated with cell count of any cell type, detected by the cell classification algorithm (see Supplementary Tables 3 and 4). Moreover, cell deformability did not differ with respect to the six different medication groups (see Supplementary Text 1).
Association between depression severity and cell deformability
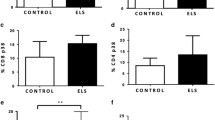
Hierarchical regression analyses with robust standard errors regarding the association of depressive symptom scores at T1 and cell deformability at T2 for each cell type are depicted in Fig. 1 and Supplementary Tables 5–9. Regarding lymphocyte deformability, model 1 and model 2 were significant (M(odel) 1: F(4, 131) = 4.495, p = .002, R² = 0.121, Adj. R² = 0.094; M 2: F(5,130) = 4.428, p < .001, R² = 0.146, Adj. R² = 0.113, ∆R² = 0.025) with BMI (ß = 0.323, p = .001, 95% Bca-CI [0.00, 0.00]) and depressive severity at T1 (ß = 0.172; p = .036, 95% Bca-CI [0.00, 0.00]) being a significant predictor for lymphocyte cell deformability in model 2. However, depressive severity at T1 did not remain significant, if applied to Holm-Bonferroni correction (pcorrected = 0.144). Model 1 and model 2 did not differ significantly, F(1,130) = 3.780, p = .054. Normal distribution of residuals in model 1 and 2 (M1: W = 0.979, p = .038; M2: W = 0.981, p = .0497) was violated as single assumption.
Ambiguous results were found regarding monocyte deformability: The overall model 1 and model 2 were not significant (M1: F(4, 131) = 1.325, p = .264, R² = 0.039, Adj. R² = 0.010; M2: F(5,130) = 2.201, p = .058, R² = 0.078, Adj. R² = 0.043, ∆R² = 0.039). However, the confounder gender (ß = 0.393, p = .042, 95% Bca-CI [0.000, 0.005]) was significant in model 1 both calculated without (p = .049) and with robust standard errors (p = .042). Moreover, in model 2, both gender (ß = 0.380, p = .040, 95% Bca-CI [0.000, 0.005]) and depressive severity at T1 (ß = 0.216; p = .007, 95% Bca-CI [0.000, 0.000]) were identified as significant predictors for monocyte deformability. Depressive severity at T1 did survive Holm-Bonferroni correction (pcorrected = 0.035). Moreover, model 1 and model 2 did differ significantly, F(1,130) = 5.522, p = .020. All assumptions were met. Exploratively, depressive severity at T1 as single predictor in a regression model led to a significant model, F(1,134) = 6.786, p = .010, R² = 0.048, Adj. R² = 0.041, ß = 0.220, SE = 0.078, p = .005, pcorrected = 0.035.
Neither a significant regression model was revealed for neutrophil deformability (M1: F(4, 131) = 1.086, p = .366, R² = 0.032, Adj. R² = 0.003. M2: F(5,130) = 0.883, p = .494, Adj. R² = -0.004, ∆R² = 0.001) nor for granulo-monocyte deformability (M1: F(4, 131) = 1.067, p = .376, R² = 0.032, Adj. R² = 0.002. M2: F(5,130) = 0.879, p = .497, R² = 0.033, Adj. R² = -0.004, ∆R² = 0.001), nor for erythrocyte deformability (M1: F(4, 131) = 1.465, p = .216, R² = 0.043, Adj. R² = 0.014. M2: F(5,130) = 1.246, p = .291, R² = 0.046, Adj. R² = 0.009, ∆R² = 0.003).
Mediation of cortisol and cortisone (T1 and T2) on immune cell deformability
By applying a mediation analyses, we examined if long-term integrated glucocorticoids mediate the association between depressive severity at T1 and immune cell deformability at T2. Despite no association between depressive severity and neutrophil, granulo-monocyte as well as erythrocyte deformability, we exploratory conducted a mediation analyses to investigate the association between gluococorticoids and cell deformability of each cell type / cluster.
No significant mediation of hair cortisol levels (T1 + T2) regarding the association of depressive severity at T1 and cell deformability was exhibited. Neither was depressive severity associated with hair cortisol levels (ß = − 0.07, p = .543) nor were hair cortisol levels associated with neutrophil (ß = 0.08, p = .456), monocyte (ß = − 0.06, p = .569), lymphocyte (ß = 0.032, p = .806), granulo-monocyte (ß = 0.04, p = .671) or erythrocyte deformability (ß = − 0.04, p = .690).
Furthermore, no significant mediation of hair cortisone levels (T1 + T2) regarding the association of depressive severity at T1 and cell deformability was revealed. Neither was depressive severity associated with hair cortisone levels (ß = − 0.07, p = .539) nor were hair cortisone levels associated with neutrophil (ß = 0.14, p = .190), monocyte (ß = 0.03, p = .734), lymphocyte (ß = 0.059, p = .621), granulo-monocyte (ß = 0.12, p = .244) or erythrocyte deformability (ß = 0.00, p = .931). A detailed overview over all models can be taken from Table 2 and Supplementary Figs. 1 and 2.
Discussion
Summary of results
Depressive symptoms at T1 were positively associated with monocyte and lymphocyte deformability at T2, supporting our first hypothesis. However, results did partly not survive correction for multiple testing or the addition of confounders. Accumulated basal glucocorticoid levels at T1 and T2 neither mediated the association between depressive symptoms and cell deformability nor were they associated with cell deformability at T2, which is not in line with the second hypothesis. Notably, there was no association between depressive symptoms and cell size. Moreover, cell count of any cell type was neither associated with depressive symptoms nor with accumulated hair glucocorticoid levels.
Integration of findings
To the authors knowledge, the present study is the first to examine the longitudinal relationship between depressive symptoms, glucocorticoid secretion and immune cell deformability in a population-based sample. The finding that elevated depressive symptomatology is associated with higher immune cell deformability one year later is supported by previously reported cross-sectional findings on the positive association between depressive disorder status, depressive symptoms and immune cell deformability [28]. Because these supporting findings originate from the same sample, it is important that further longitudinal as well as experimental studies replicate and extend these associations.
The observed significant positive associations between depressive symptoms at T1 and immune cell deformability at T2 for monocytes and lymphocytes in the entire sample and, as reported in Walther et al. (2022), for monocytes, neutrophils, and granulo-monocytes in the lifetime PDD and healthy control subsample, suggests these immune cells to be the most sensitive cells to react to depressive symptomatology with physical changes. These changes might be due to alterations in membrane-forming lipids, which have already been linked to DD in the central nervous system [67]. Moreover, since these cells are rich in glucocorticoid receptors [68,69,70,71], and HPA-axis alterations are considered a pathophysiological landmark of DD [14, 40], it would be reasonable to assume that changes in glucocorticoid secretion might also affect immune cell status and deformability. Indeed, previous research indicates that increased glucocorticoid levels led to increased immune cell counts due to cell demargination from the vessel walls, which was mediated by cellular softening [29]. However, the present study did not reveal any direct associations between accumulated glucocorticoid levels at T1 and T2 with cell deformability at T2 or with cell count at T2. Notably, we did not observe a positive association between levels of circulating immune cells and depressive symptoms in the present study (see Supplementary Table 2), although this was previously reported by another study for classificatory-categorical depression cases and depressive symptom severity [49]. In contrast to a previous report [43], no direct association between hair glucocorticoid levels and depressive symptoms emerged (see Table 4).
Our results suggest that accumulated long-term glucocorticoid concentrations over a one-year period do not affect immune cell structure and stability. Immune cells might respond only to short-term and synchronized increased glucocorticoid concentrations by reorganizing the cytoskeleton, which affects cell deformability directly as previous studies showed using dexamethasone administration [29, 72]. Hence, our assumption that elevated long-term glucocorticoid concentrations can cause increased permeation through the cell membrane affecting the lipidome and the cell structure leading to membrane destabilization and bending [20], and further to increased cell deformability could not be supported by our findings.
Compared to our case-control analyses in Walther et al. (2022), we did not detect increased neutrophil deformability with higher depression severity. Given the persistence of depressive symptoms in PDD over two years [3], associations with neutrophil cell deformability seem to be more detectable than in a snapshot regarding the presence of depressive symptoms within the last weeks. It is also likely that in PDD, the HPA axis shows hyperactivity with increased glucocorticoid output, as there is less depressive symptomatology but over a longer period of time than for example in MDD. In MDD, the HPA axis appears to enter exhaustion at some point, at which point a shift from increased to decreased levels of glucocorticoids are more likely [14, 46, 47, 73]. Thus, many reports also identify reduced hair cortisol levels or more complex patterns in MDD, especially in conjunction with experienced trauma [45, 74,75,76]. Therefore, future studies examining the relationship between DD or depressive symptoms, glucocorticoid output and immune cell deformability should aim to assess the time period since when individuals are suffering from depressive symptomatology as well as childhood maltreatment or trauma experience to better disentangle these seemingly complex associations.
Overall, the present study provides new insights into the pathophysiology of depressive symptoms, assuming more deformed immune cells, especially monocytes and lymphocytes over a small population-based sample. As long-term glucocorticoid output does not seem to be the underlying mechanism for the association of depressive symptoms and cell deformability, research needs to investigate whether further processes consistently linked to DD such as low-grade inflammation [15, 18, 77] or oxidative stress [30] might be additional factors contributing to cytoskeletal alterations and membrane destabilization [78, 79] resulting in higher immune cell deformability in DD or individuals suffering from depressive symptoms. This might be because, similar to increased glucocorticoid secretion, an inflammatory state leads to demargination of blood cells from the vessel walls into the circulation, with cell deformability being increased by the migration process [29]. Additionally, previous research indicates that an experimentally induced immune activation with lipopolysaccharide increased monocyte deformability [33], highlighting chronic low-grade inflammation in depressed individuals as potential agent for increased immune cell deformability. These assumptions are supported by findings of individuals with increased inflammatory signaling, such as healthy individuals after inhalation of lipopolysaccharide from E. coli, or individuals suffering from acute lung injury, viral respiratory infections, or Epstein-Barr virus infections, show increased deformability of neutrophils, monocytes, or lymphocytes [25]. Moreover, neutrophils of individuals in the acute phase of COVID-19 infection showed higher deformability, which was still observable also seven months after the acute infection symptoms [24], suggesting that long-term HPA-axis or inflammatory alterations may underlie immune cell deformability in DD.
It is important to mention that self-reported medication intake was not associated with immune cell deformability. As subjects were not drug naïve, we tested the association of different medication groups with cell deformability, such as psychopharmaceutical medication, antihypertensive drugs, and thyroid dysfunction medication. No association between any medication group and cell deformability was identified. Also, associations between depressive symptomatology and cell deformability cannot be traced back to cell size, as higher depression severity and cell size of immune cells were not associated with each other.
Strengths and limitations
Some limitations must be taken into account when interpreting our results. The most important limitation is the single measurement of cell deformability at T2. To investigate the predictive value of depressive symptoms and accumulated glucocorticoid concentrations over a one-year period on cell deformability, we measured cell deformability as an outcome measure at T2. However, analyses of changes in cell deformability in relation to changes in depressive symptoms or glucocorticoid concentrations would also be important to investigate. Furthermore, with such a study design, the overall stability of cell deformability markers could be recorded. A related point is the single assessment of the diagnostic groups using a clinical interview at T2. Since we intended to capture group differences in cell deformability [28], the clinical diagnostics as well as the cell deformability measurement had to be performed at the same time point in order to avoid possible time effects. However, in a follow-up project, clinical diagnostics will also be performed at two time points together with cell deformability measurement. Nevertheless, our findings provide first evidence that depressive symptoms are related to immune cell deformability one year later, which are not mediated by long-term glucocorticoid secretion. Following on from this, however, it should be noted that other important systemic markers have not been investigated in the present study. Thus, it will be important in future studies to explore potential associations between inflammatory markers such as Interleukin-6 or C-reactive protein and immune cell deformability. In addition, the present sample of 136 participants might be underpowered to detect small to moderate effects, so only relatively large effects were probably identified in the present study further explaining the partly non-robust relationship between depressive symptoms and immune cell deformability. Finally, as gender differences and BMI partly play a role in this study, larger mixed samples or samples that focus only on women, men, or gender-diverse individuals need to be further investigated.
Conclusions
Taken together, this study represents the first longitudinal investigation of the association between depressive symptoms, glucocorticoid concentrations, and peripheral immune cell deformability in a population-based sample. Our results suggest that higher levels of depressive symptoms are longitudinally associated with higher immune cell deformability, particularly in monocytes and lymphocytes. However, results have to be interpreted with caution due to lack of stability. Moreover, we could not confirm, that accumulated glucocorticoid concentrations over one year lead to cytoskeletal changes and cell membrane destabilization causing overall increased immune cell deformability. Increased immune cell deformability may either represent an adapted state of a regulatory immune response or a gradual loss of immune cell functionality, so that, for example, an adequate immune response is impaired or membrane function is compromised presenting a potentially underlying mechanism causing or maintaining depressive symptoms.
Data and code availability
The anonymized data and code will be made available to all interested parties upon request.
References
World Health Organization (2017) Depression and other common mental disorders: global health estimates. World Health Organization
Vos T, Lim SS, Abbafati C et al (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet 396:1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5™, 5th edn. American Psychiatric Publishing, Inc., Arlington, VA, US
Hasin DS, Sarvet AL, Meyers JL et al (2018) Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75:336. https://doi.org/10.1001/jamapsychiatry.2017.4602
Vos T, Abajobir AA, Abate KH et al (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet 390:1211–1259. https://doi.org/10.1016/S0140-6736(17)32154-2
Munder T, Flückiger C, Leichsenring F et al (2018) Is psychotherapy effective? A re-analysis of treatments for depression. Epidemiol Psychiatr Sci 28:268–274. https://doi.org/10.1017/S2045796018000355
Munkholm K, Paludan-Müller AS, Boesen K (2019) Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta-analysis. BMJ Open 9:e024886. https://doi.org/10.1136/bmjopen-2018-024886
Guidi J, Fava GA, Fava M, Papakostas GI (2011) Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: a preliminary meta-analysis. Psychol Med 41:321–331. https://doi.org/10.1017/S0033291710000826
McLachlan G (2018) Treatment resistant depression: what are the options? https://doi.org/10.1136/bmj.k5354. BMJ k5354
Rush AJ, Trivedi MH, Wisniewski SR et al (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. https://doi.org/10.1176/ajp.2006.163.11.1905
Biselli T, Lange SS, Sablottny L et al (2021) Optogenetic and chemogenetic insights into the neurocircuitry of depression-like behaviour: a systematic review. Eur J Neurosci 53:9–38. https://doi.org/10.1111/ejn.14603
Fiacco S, Walther A, Ehlert U (2019) Steroid secretion in healthy aging. Psychoneuroendocrinology 105:64–78. https://doi.org/10.1016/j.psyneuen.2018.09.035
Otte C, Gold SM, Penninx BW et al (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065. https://doi.org/10.1038/nrdp.2016.65
Rothe N, Steffen J, Penz M et al (2020) Examination of peripheral basal and reactive cortisol levels in major depressive disorder and the burnout syndrome: a systematic review. Neurosci Biobehavioral Reviews 114:232–270. https://doi.org/10.1016/j.neubiorev.2020.02.024
Iob E, Kirschbaum C, Steptoe A (2020) Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry 25:1130–1140. https://doi.org/10.1038/s41380-019-0501-6
Lopez-Vilchez I, Diaz-Ricart M, Navarro V et al (2016) Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Transl Psychiatry 6:e886. https://doi.org/10.1038/tp.2016.156
Moylan S, Maes M, Wray NR, Berk M (2013) The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18:595–606. https://doi.org/10.1038/mp.2012.33
Pariante CM (2017) Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur Neuropsychopharmacol 27:554–559. https://doi.org/10.1016/j.euroneuro.2017.04.001
Rodrigues R, Petersen RB, Perry G (2014) Parallels between major depressive disorder and Alzheimer’s disease: role of oxidative stress and genetic vulnerability. Cell Mol Neurobiol 34:925–949. https://doi.org/10.1007/s10571-014-0074-5
Walther A, Cannistraci CV, Simons K et al (2018) Lipidomics in major depressive disorder. Front Psychiatry 9:459. https://doi.org/10.3389/fpsyt.2018.00459
Wolkowitz OM, Epel ES, Reus VI, Mellon SH (2010) Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety 27:327–338. https://doi.org/10.1002/da.20686
Otto O, Rosendahl P, Mietke A et al (2015) Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat Methods 12:199–202. https://doi.org/10.1038/nmeth.3281
Rosenbluth MJ, Lam WA, Fletcher DA (2008) Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab Chip 8:1062–1070. https://doi.org/10.1039/b802931h
Kubánková M, Hohberger B, Hoffmanns J et al (2021) Physical phenotype of blood cells is altered in COVID-19. Biophys J 120:2838–2847. https://doi.org/10.1016/j.bpj.2021.05.025
Toepfner N, Herold C, Otto O et al (2018) Detection of human disease conditions by single-cell morpho-rheological phenotyping of blood. eLife 7:e29213. https://doi.org/10.7554/eLife.29213
Bashant KR, Vassallo A, Herold C et al (2019) Real-time deformability cytometry reveals sequential contraction and expansion during neutrophil priming. J Leukoc Biol 105:1143–1153. https://doi.org/10.1002/JLB.MA0718-295RR
Bashant KR, Toepfner N, Day CJ et al (2020) The mechanics of myeloid cells. Biol Cell 112:103–112. https://doi.org/10.1111/boc.201900084
Walther A, Mackens-Kiani A, Eder J et al (2022) Depressive disorders are associated with increased peripheral blood cell deformability: a cross-sectional case-control study (Mood-Morph). Transl Psychiatry 12:1–12. https://doi.org/10.1038/s41398-022-01911-3
Fay ME, Myers DR, Kumar A et al (2016) Cellular softening mediates leukocyte demargination and trafficking, thereby increasing clinical blood counts. Proc Natl Acad Sci USA 113:1987–1992. https://doi.org/10.1073/pnas.1508920113
Moylan S, Berk M, Dean OM et al (2014) Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45:46–62. https://doi.org/10.1016/j.neubiorev.2014.05.007
Ekpenyong AE, Whyte G, Chalut K et al (2012) Viscoelastic properties of differentiating blood cells are fate- and function-dependent. PLoS ONE 7:e45237. https://doi.org/10.1371/journal.pone.0045237
Lam WA, Rosenbluth MJ, Fletcher DA (2008) Increased leukaemia cell stiffness is associated with symptoms of leucostasis in paediatric acute lymphoblastic leukaemia. Br J Haematol 142:497–501. https://doi.org/10.1111/j.1365-2141.2008.07219.x
Ravetto A, Wyss HM, Anderson PD et al (2014) Monocytic cells become less compressible but more deformable upon activation. PLoS ONE 9:e92814. https://doi.org/10.1371/journal.pone.0092814
Jasenovec T, Radosinska D, Celusakova H et al (2019) Erythrocyte deformability in children with autism spectrum disorder: correlation with clinical features. Physiol Res 68:S307–S313. https://doi.org/10.33549/physiolres.934349
Saha AK, Schmidt BR, Wilhelmy J et al (2019) Red blood cell deformability is diminished in patients with chronic fatigue syndrome. Clin Hemorheol Microcirc 71:113–116. https://doi.org/10.3233/CH-180469
Kräter M, Abuhattum S, Soteriou D et al (2021) AIDeveloper: deep learning image classification in life science and beyond. Adv Sci 8:2003743. https://doi.org/10.1002/advs.202003743
Nawaz AA, Urbanska M, Herbig M et al (2020) Intelligent image-based deformation-assisted cell sorting with molecular specificity. Nat Methods 17:595–599. https://doi.org/10.1038/s41592-020-0831-y
Urbanska M, Muñoz HE, Shaw Bagnall J et al (2020) A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat Methods 17:587–593. https://doi.org/10.1038/s41592-020-0818-8
Wu P-H, Aroush DR-B, Asnacios A et al (2018) A comparison of methods to assess cell mechanical properties. Nat Methods 15:491–498. https://doi.org/10.1038/s41592-018-0015-1
Stetler C, Miller GE (2011) Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73:114–126. https://doi.org/10.1097/PSY.0b013e31820ad12b
Miller R, Kirschbaum C (2019) Cultures under stress: a cross-national meta-analysis of cortisol responses to the Trier Social Stress Test and their association with anxiety-related value orientations and internalizing mental disorders. Psychoneuroendocrinology 105:147–154. https://doi.org/10.1016/j.psyneuen.2018.12.236
Zorn JV, Schür RR, Boks MP et al (2017) Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology 77:25–36. https://doi.org/10.1016/j.psyneuen.2016.11.036
Gerritsen L, Staufenbiel SM, Penninx BWJH et al (2019) Long-term glucocorticoid levels measured in hair in patients with depressive and anxiety disorders. Psychoneuroendocrinology 101:246–252. https://doi.org/10.1016/j.psyneuen.2018.11.019
Rothe N, Vogel S, Schmelzer K et al (2021) The moderating effect of cortisol and dehydroepiandrosterone on the relation between sleep and depression or burnout. Compr Psychoneuroendocrinology 7:100051. https://doi.org/10.1016/j.cpnec.2021.100051
Stalder T, Steudte-Schmiedgen S, Alexander N et al (2017) Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 77:261–274. https://doi.org/10.1016/j.psyneuen.2016.12.017
Steudte-Schmiedgen S, Wichmann S, Stalder T et al (2017) Hair cortisol concentrations and cortisol stress reactivity in generalized anxiety disorder, major depression and their comorbidity. J Psychiatr Res 84:184–190. https://doi.org/10.1016/j.jpsychires.2016.09.024
Walther A, Kirschbaum C, Wehrli S et al (2023) Depressive symptoms are negatively associated with hair N-arachidonoylethanolamine (anandamide) levels: a cross-lagged panel analysis of four annual assessment waves examining hair endocannabinoids and cortisol. Prog Neuropsychopharmacol Biol Psychiatry 121:110658. https://doi.org/10.1016/j.pnpbp.2022.110658
Kim J-K, Shin YJ, Ha LJ et al (2019) Unraveling the mechanobiology of immune system. Adv Healthc Mater 8:e1801332. https://doi.org/10.1002/adhm.201801332
Lynall M-E, Turner L, Bhatti J et al (2020) Peripheral blood cell-stratified subgroups of inflamed depression. Biol Psychiatry 88:185–196. https://doi.org/10.1016/j.biopsych.2019.11.017
Demirkan A, Isaacs A, Ugocsai P et al (2013) Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family-based lipidomics study. J Psychiatr Res 47:357–362. https://doi.org/10.1016/j.jpsychires.2012.11.001
Knowles EEM, Huynh K, Meikle PJ et al (2017) The lipidome in major depressive disorder: Shared genetic influence for ether-phosphatidylcholines, a plasma-based phenotype related to inflammation, and disease risk. Eur Psychiatry 43:44–50. https://doi.org/10.1016/j.eurpsy.2017.02.479
Liu X, Li J, Zheng P et al (2016) Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal Bioanal Chem 408:6497–6507. https://doi.org/10.1007/s00216-016-9768-5
Dantzer R (2009) Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 29:247–264. https://doi.org/10.1016/j.iac.2009.02.002
Penz M, Wekenborg MK, Pieper L et al (2018) The Dresden Burnout Study: protocol of a prospective cohort study for the bio-psychological investigation of burnout. Int J Methods Psychiatr Res 27:e1613. https://doi.org/10.1002/mpr.1613
Kroenke K, Spitzer RL, Williams JBW (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
Ware J, Kosinski M, Keller SD (1996) A 12-Item short-form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233. https://doi.org/10.1097/00005650-199603000-00003
He C, Levis B, Riehm KE et al (2020) The accuracy of the Patient Health Questionnaire-9 algorithm for screening to detect major depression: an individual participant data meta-analysis. Psychother Psychosom 89:25–37. https://doi.org/10.1159/000502294
Levis B, Sun Y, He C et al (2020) Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: systematic review and meta-analysis. JAMA 323:2290. https://doi.org/10.1001/jama.2020.6504
Hoyer J, Voss C, Strehle J et al (2020) Test-retest reliability of the computer-assisted DIA-X-5 interview for mental disorders. BMC Psychiatry 20:280. https://doi.org/10.1186/s12888-020-02653-6
Gao W, Stalder T, Foley P et al (2013) Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci 928:1–8. https://doi.org/10.1016/j.jchromb.2013.03.008
Gao W, Kirschbaum C, Grass J, Stalder T (2016) LC-MS based analysis of endogenous steroid hormones in human hair. J Steroid Biochem Mol Biol 162:92–99. https://doi.org/10.1016/j.jsbmb.2015.12.022
Wennig R (2000) Potential problems with the interpretation of hair analysis results. Forensic Sci Int 107:5–12. https://doi.org/10.1016/s0379-0738(99)00146-2
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Hayes AF (2022) Introduction to mediation, moderation, and conditional process analysis: a regression-based approach, Third edition. The Guilford Press, New York London
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Croux C, Dehon C (2010) Influence functions of the Spearman and Kendall correlation measures. Stat Methods Appl 19:497–515. https://doi.org/10.1007/s10260-010-0142-z
Müller CP, Reichel M, Mühle C et al (2015) Brain membrane lipids in major depression and anxiety disorders. Biochimica et Biophysica Acta (BBA) - molecular and cell. Biology Lipids 1851:1052–1065. https://doi.org/10.1016/j.bbalip.2014.12.014
Goecke IA, Alvarez C, Henríquez J et al (2007) Methotrexate regulates the expression of glucocorticoid receptor alpha and beta isoforms in normal human peripheral mononuclear cells and human lymphocyte cell lines in vitro. Mol Immunol 44:2115–2123. https://doi.org/10.1016/j.molimm.2006.07.303
Heiske A, Jesberg J, Krieg J-C, Vedder H (2003) Differential effects of antidepressants on glucocorticoid receptors in human primary blood cells and human monocytic U-937 cells. Neuropsychopharmacology 28:807–817. https://doi.org/10.1038/sj.npp.1300056
Li L-B, Leung DYM, Hall CF, Goleva E (2006) Divergent expression and function of glucocorticoid receptor β in human monocytes and T cells. J Leukoc Biol 79:818–827. https://doi.org/10.1189/jlb.0805466
Lu KD, Radom-Aizik S, Haddad F et al (2017) Glucocorticoid receptor expression on circulating leukocytes differs between healthy male and female adults. J Clin Transl Sci 1:108–114. https://doi.org/10.1017/cts.2016.20
Ronchetti S, Ricci E, Migliorati G et al (2018) How glucocorticoids affect the neutrophil life. Int J Mol Sci 19:4090. https://doi.org/10.3390/ijms19124090
Steudte-Schmiedgen S, Kirschbaum C, Alexander N, Stalder T (2016) An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: insight from recent hair cortisol findings. Neurosci Biobehav Rev 69:124–135. https://doi.org/10.1016/j.neubiorev.2016.07.015
Cantave CY, Ouellet-Morin I, Giguère C-É et al (2022) The association of childhood maltreatment, sex, and hair cortisol concentrations with trajectories of depressive and anxious symptoms among adult psychiatric inpatients. Psychosom Med 84:20–28. https://doi.org/10.1097/PSY.0000000000001016
Hinkelmann K, Muhtz C, Dettenborn L et al (2013) Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biol Psychiatry 74:e15–17. https://doi.org/10.1016/j.biopsych.2013.04.021
Psarraki EE, Kokka I, Bacopoulou F et al (2021) Is there a relation between major depression and hair cortisol? A systematic review and meta-analysis. Psychoneuroendocrinology 124:105098. https://doi.org/10.1016/j.psyneuen.2020.105098
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. https://doi.org/10.1038/mp.2016.3
Wilson C, González-Billault C (2015) Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front Cell Neurosci 9:381. https://doi.org/10.3389/fncel.2015.00381
Wong GT-H, Chang RC-C, Law AC-K (2013) A breach in the scaffold: the possible role of cytoskeleton dysfunction in the pathogenesis of major depression. Ageing Res Rev 12:67–75. https://doi.org/10.1016/j.arr.2012.08.004
Funding
Open access funding provided by University of Zurich. Research pool TU Dresden (F-004242-552-848-1040103) awarded to AW. Faculty of Psychology of the TU Dresden (MK201911) awarded to AW. Dresden University of Technology Institutional Strategy (“The Synergetic University”), which is funded by the Excellence Initiative of the German Federal and State Governments awarded to CK. Swiss National Science Foundation (PZPGP1_201757) awarded to AW. The funding sources are national and university funding sources and had no influence on the writing of the manuscript or the decision to submit it for publication. None of the authors received financial incentives from industrial companies to write this publication. The corresponding author had full access to all study data and had the final responsibility for the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
AW and MK designed the Mood-Morph study. AW, JE and MK performed experiments and data analysis. CK designed the longitudinal study. AW and JE wrote the first draft of the manuscript. WG, MW, MP, NR performed experiments. CK, JG, MK, LW provided funding, and infrastructure/equipment. All authors edited previous versions of the manuscript, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no actual or potential conflict of interest to declare, including any financial, personal, or other relationships with other people or organisations within 3 years of beginning the submitted work that could influence or bias their work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eder, J., Kräter, M., Kirschbaum, C. et al. Longitudinal associations between depressive symptoms and cell deformability: do glucocorticoids play a role?. Eur Arch Psychiatry Clin Neurosci (2024). https://doi.org/10.1007/s00406-024-01902-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-024-01902-z