Abstract
Breast cancer is one of the most prevalent and serious types of cancer globally. Previous literature has shown that women with mental illness may have an increased risk of breast cancer, however whether this risk is associated with the use of psychotropic drugs has yet to be elucidated. This study aimed to assess such risk among women with major depressive disorder (MDD) and bipolar disorder (BD). A nested case–control study design was used with data obtained from the Taiwan National Health Insurance Research Database. Logistic regression analysis with adjustments for demographic characteristics, medical and mental comorbidities, and all-cause clinical visits was performed to estimate the risk of breast cancer according to the cumulative defined daily dose (cDDD) of psychotropic drugs. The study included 1564 women with MDD or BD who had breast cancer, and 15,540 women with MDD or BD who did not have breast cancer. After adjusting for important confounders, the long-term use of valproic acid (odds ratio, 95% confidence interval: 0.58, 0.39–0.56, cDDD ≥ 365), citalopram (0.58, 0.37–0.91, cDDD 180–365), and sertraline (0.77, 0.61–0.91, cDDD ≥ 365) was associated with a lower risk of breast cancer compared to a cDDD < 30. The short-term use of fluvoxamine (0.82, 0.69–0.96, cDDD 30–180), olanzapine (0.54, 0.33–0.89, cDDD 30–179), risperidone (0.7, 0.51–0.98, cDDD 30–179), and chlorpromazine (0.48, 0.25–0.90, cDDD 30–179) was associated with a lower risk of breast cancer. We found no evidence of an increased risk of breast cancer in patients with MDD or BD receiving psychotropic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is common among women worldwide [1], and previous research has suggested an increased risk of cancer mortality in patients with mental illness [2]. Despite this increased risk, the rate of cancer screening in such patients is often lower compared with the general population [3]. Several studies have also reported the under-utilization of preventive services, limited access to treatment, and challenges with adherence to cancer treatment in patients with mental illness [4, 5].
Findings regarding the association between cancer and major depressive disorder (MDD) and bipolar disorder (BD) are still controversial. One genetic study reported a significant risk of breast cancer in patients with MDD [6], while another study did not [7]. Due to the complicated relationship between breast cancer and mental illness, researchers have investigated the potential role of psychotropic drugs in this association, including antipsychotics, mood stabilizers, and antidepressants [8,9,10,11]. However, evidence from these studies is limited or inconclusive. Two large observational studies reported a significant risk of breast cancer associated with prolactin-related antipsychotics [10, 12], however whether prolactin-related antipsychotics stimulate breast cancer cell growth remains inconclusive [13, 14]. A critical review of human prospective studies showed equivocal results, with risk ratios ranging from 0.70 to 1.9 for premenopausal women and 0.76 to 2.03 for postmenopausal women [15]. Controversial findings have also been reported in the association between antidepressants and breast cancer risk. Several studies suggested that antidepressant use was linked to a 50–75% increased risk of breast cancer [16, 17], whereas another population-based study found no evidence of such an association [8]. As for other psychotropic drugs, numerous in vitro and in vivo preclinical studies have suggested that anticonvulsant drugs significantly inhibit cancer cell proliferation by modulating multiple signaling pathways. However, these effects have been demonstrated only in in vitro experiments using valproic acid [11], and evidence for carbamazepine [18] and lamotrigine [19] remains at the preclinical stage.
Aim of the current study
There is currently insufficient evidence regarding the association between breast cancer and psychotropic drugs, especially mood stabilizers. In addition, the inconsistent results regarding antipsychotics and antidepressants highlights the need for further studies to better validate these findings. Besides research focusing on participants with schizophrenia [10], the association between the use of psychotropic agents and the risk of breast cancer has rarely been investigated among patients with BD or MDD. Given the gaps in previous evidence, the aim of this study was to comprehensively assess the risk of breast cancer associated with the prescription of psychotropic drugs (antipsychotics, antidepressants, and mood stabilizers) among a large cohort of patients with MDD and BD. Investigating this association could be helpful in clarifying the complex etiology of breast cancer risk among patients with severe mental illness (SMI), as well as providing healthcare workers with clinical implications for making informed decisions about the use of psychotropic drugs and discussing treatment options with patients. Our hypothesis is that certain psychotropic drugs may be associated with the risk of breast cancer.
Methods
Data source
The Taiwan National Health Research Institute oversees the National Health Insurance Research Database (NHIRD), which is available for scientific and research purposes [20, 21]. The NHIRD anonymizes individual medical records to protect patient privacy. In this study, we linked two databases: the specialized dataset of mental disorders, which includes all medical records (mental and non-mental) of insured individuals with mental disorders, and the Catastrophic Illness database, which includes diagnoses of catastrophic illnesses (such as malignant cancers) and the diagnosis date [22]. In Taiwan, the diagnosis of malignant cancers is reviewed by commissioned expert panels, and patients diagnosed with cancer are exempt from medical copayments. The diagnostic codes used in the NHIRD between 1996 and 2011 were based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The Institutional Review Board of Taipei Veterans General Hospital approved the study protocol, and the requirement for informed consent was waived. The NHIRD has been used in many epidemiological studies in Taiwan [23,24,25,26].
Study participants
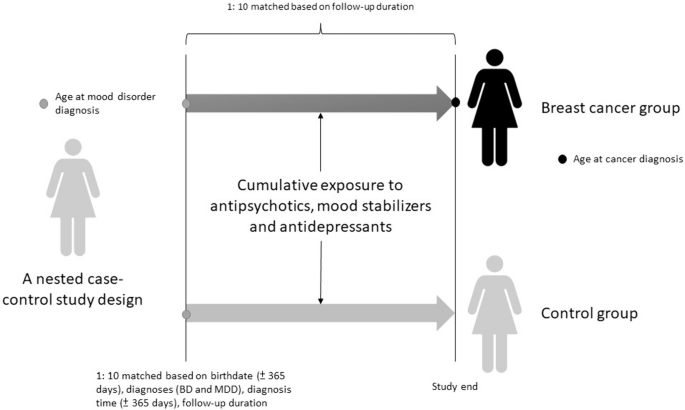
The current study was designed as a nested case–control study. The study cohort consisted of women aged ≥ 20 years with psychiatrist-diagnosed BD (ICD-9-CM codes: 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.7x, 296.80, 296.81, 296.89) or MDD (ICD-9-CM codes: 296.2 × and 296.3x) who had no history of any malignant cancer prior to 2001 and up to 2011. The date of the diagnosis of BD or MDD was defined as the enrollment date, while the date of the diagnosis of breast cancer was defined as the endpoint date. The follow-up period was calculated from the enrollment date to the endpoint date. Cases were those who were subsequently diagnosed with malignant breast cancer (ICD-9-CM code: 174) from enrollment to the end of 2011 or death. Controls were selected from the cohort with BD or MDD but without a diagnosis of any malignant cancer. The controls were selected in a 10:1 ratio with the study cases based on birthdate (± 365 days), diagnosis (BD and MDD), diagnosis date (± 365 days), follow-up duration, medical and mental comorbidities (hypertension, dyslipidemia, diabetes mellitus, obesity, smoking, and alcohol and substance use disorders), income, and residence. A 10:1 ratio was used to enhance the statistical power and to ensure an adequate number of cases with BD or MDD for stratified analyses [27]. Income level (levels 1–3 per month: ≤ 19,100 New Taiwan Dollars [NTD], 19,001 ~ 42,000 NTD, and > 42,000 NTD) and urbanization level of residence (levels 1–5, most to least urbanized) were assessed as proxies for healthcare availability in Taiwan [28]. Additionally, data on Charlson Comorbidity Index (CCI) and all-cause clinical visits were obtained for the study and matched-control cohorts. The CCI, consisting of 22 physical conditions (cancer was excluded in the current study), was assessed to determine the systemic health conditions of the enrolled women [29]. In the CCI, each physical condition has an associated weight (from 1 to 6) based on the adjusted risk of mortality. The sum of all the weights yields a single comorbidity score for a patient. A score of zero indicates the absence of comorbidities, and the higher the score, the more likely the outcome will result in mortality or greater use of healthcare resources. The study design is shown in Fig. 1.
Exposure to psychotropic medications
Information on prescribed drugs, including the World Health Organization Anatomical Therapeutic Chemical classification system, drug dosage, supply days, and number of dispensed drug pills, was extracted from the NHIRD. The World Health Organization’s defined daily dose (DDD) is a unit for measuring a prescribed amount of drug. The DDD is the assumed average maintenance dose per day of a drug consumed for its main indication. We calculated the cumulative DDD (cDDD) of all first-generation antipsychotics (FGAs; chlorpromazine, clotiapine, flupentixol, fluphenazine, haloperidol, loxapine, and sulpiride), second-generation antipsychotics (SGAs; aripiprazole, risperidone, olanzapine, amisulpride, ziprasidone, clozapine, and quetiapine), mood stabilizers (lithium, carbamazepine, oxcarbazepine, valproate, lamotrigine, topiramate, and gabapentin), and antidepressants (fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, escitalopram, venlafaxine, duloxetine, milnacipran, bupropion, and mirtazapine) during the follow-up period. Based on the cDDD [30,31,32], medication use patterns were classified into four subgroups: cDDD of < 30, cDDD of 30 to 179, cDDD of 180 to 364, and cDDD of ≥ 365.
Statistical analysis
The independent t-test was used to compare continuous variables between groups, and Pearson’s χ2 test was used for nominal variables, as appropriate. Logistic regression analyses were performed with adjustments for demographic characteristics, medical and mental comorbidities, CCI scores, and all-cause clinical visits to calculate the odds ratio (OR) and 95% confidence interval (CI) of the association between psychotropic medication use (cDDD categories: < 30, 30–179, 180–364, and ≥ 365) and subsequent breast cancer risk. The psychotropic medications included FGAs, SGAs, mood stabilizers, and antidepressants. Previous studies have shown a slightly increased risk (incidence rate ratios ranging from 1.05 to 1.20) of breast cancer among women with major affective disorders, including BD and MDD [33,34,35]. Using G-power to estimate the sample size with an α error probability of 0.05 and a 1-β error probability (power) of 0.95, the optimal sample size was ≥ 10,905 at a rate ratio of 1.05, and ≥ 780 at a rate ratio of 1.20. A two-tailed p value < 0.05 was considered statistically significant. All data processing and statistical analyses were performed using Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC).
Data availability statement
The NHIRD is released and audited by the Department of Health and Bureau of the NHI Program for scientific research purposes (https://nhird.nhri.org.tw/). The NHIRD can be obtained through a formal application regulated by the Department of Health and Bureau of the NHI Program.
Results
Demographic and clinical information
A total of 1564 cases (women with BD/MDD and breast cancer; BD = 283 and MDD = 1281) and 15,540 matched controls (women with BD/MDD without breast cancer) were included in the current study (Table 1). Compared to the control group, the breast cancer group had a significantly higher CCI score (2.68 vs 1.13, p < 0.001) and a higher number of all-cause clinical visits (19.62 vs 16.84, p < 0.001). However, the two groups did not significantly differ in terms of medical comorbidities, mental comorbidities, and demographic characteristics (Table 1). The distribution of antipsychotic prescriptions is listed in Supplementary Table 1.
Risk of breast cancer in different groups of exposure to mood stabilizers and antidepressants in the patients with BD or MDD
After adjusting for date of birth, demographic characteristics, date of diagnosis, follow-up duration, medical and mental comorbidities, CCI score, and all-cause clinical visits (Table 2), the group with a cDDD of ≥ 365 for all mood stabilizers was associated with a lower risk of breast cancer than the group with a cDDD of < 30 for all mood stabilizers (reported as OR with 95% CI 0.75; 0.59–0.95). Specifically, the group with a cDDD ≥ 365 of valproic acid was associated with a lower risk of breast cancer than the group with a cDDD of < 30 of valproic acid (0.58; 0.39–0.56). The group with a cDDD of ≥ 365 for all antidepressants was associated with a lower risk of breast cancer than the group with a cDDD of < 30 for all antidepressants (0.79; 0.68–0.92). The group with a cDDD of 180 to 364 of citalopram was associated with a lower risk of breast cancer than the group with a cDDD of < 30 of citalopram (0.58; 0.37–0.91). The group with a cDDD of 30 to 179 of fluvoxamine was associated with a lower risk of breast cancer than the group with a cDDD of < 30 of fluvoxamine (0.58; 0.37–0.91). The group with a cDDD of ≥ 365 of sertraline (0.77; 0.61–0.97) and the group with a cDDD of 30 to 179 of sertraline (0.68; 0.55–0.82) were associated with lower risks of breast cancer than the group with a cDDD of < 30 of sertraline.
Risk of breast cancer in different groups of exposure to antipsychotics in the patents with BD or MDD
In terms of SGAs, the group with a cDDD of 30–179 for all SGAs had a lower risk of breast cancer compared to the group with a cDDD of < 30 for all SGAs (0.58; 0.44–0.75). The group with a cDDD of 30–179 of olanzapine had a lower risk of breast cancer compared to the group with a cDDD of < 30 of olanzapine (0.54; 0.33–0.89). The group with a cDDD of 30–179 of risperidone had a lower risk of breast cancer compared to the group with a cDDD of < 30 of risperidone (0.70; 0.51–0.98). The group with a cDDD of 30–179 of chlorpromazine had a lower risk of breast cancer compared to the group with a cDDD of < 30 of chlorpromazine (0.48; 0.25–0.90). However, the group with a cDDD of 180–364 of ziprasidone had a higher risk of breast cancer compared to the group with a cDDD of < 30 of ziprasidone (4.70; 1.47–15.07) (see Table 3).
Discussion
Main findings of the current study
The results of this study demonstrated that overall, the use of mood stabilizers, antidepressants, and SGAs was associated with a reduced risk of breast cancer among female patients with BD or MDD. Specifically, of the mood stabilizers, the long-term use of valproic acid was linked to a decreased risk of breast cancer. In addition, the long-term use of citalopram or sertraline was associated with a lower risk of breast cancer, while the short-term use of fluvoxamine, chlorpromazine, olanzapine, or risperidone was associated with a lower risk of breast cancer. However, the use of ziprasidone with a cDDD of 180 to 364 (not long-term use) was linked to an increased risk of breast cancer compared to a cDDD of < 30. In summary, we found that the use of the aforementioned psychotropic agents except ziprasidone may reduce the risk of breast cancer in patients with BD and MDD.
Decreased risk of breast cancer with the use of mood stabilizers and antidepressants
Our results demonstrated that the long-term use of mood stabilizers was associated with a reduced risk of breast cancer in the study patients, and in particular the long-term use of valproic acid over the short-term use. A potential mechanism for this effect is that valproic acid, a broad class I histone deacetylase (HDAC) inhibitor, may decrease the expression of the pyruvate kinase M2 isoform, leading to inhibited cell proliferation and reduced colony formation in breast cancer cells [36]. Several in vitro and in vivo preclinical studies have suggested that valproic acid significantly inhibits cancer cell proliferation and metastasis by modulating multiple signaling pathways, tumor immune response, and cell cycle arrest through HDAC inhibition [11, 37].
The debate surrounding antidepressant treatment and increased risk of breast cancer has persisted for many years. While preliminary data have suggested a risk of breast cancer in users of antidepressants [16, 17], other epidemiological studies using nationwide databases [8] and large prospective surveys (Women’s Health Initiative Observational Study) [38] have found no such association. A possible etiology linking antidepressants to the risk of breast cancer may be through the effect of prolactin. Treatment with selective serotonin reuptake inhibitors (SSRIs) may increase circulating prolactin levels [39, 40], which could potentially increase the risk of breast cancer by stimulating cellular proliferation, differentiation, and angiogenesis [13]. However, recent evidence does not support the association between SSRIs and the risk of breast cancer [41], and a recent cohort study did not observe increased prolactin levels among women using antidepressants or SSRIs [42]. The findings of such an association in previous studies may be due to relatively small sample sizes or confounding factors. In contrast to previous findings, we found that the long-term use of antidepressants including citalopram, fluvoxamine, and sertraline may be associated with a decreased risk of breast cancer. The mechanism for this effect may be explained by the anti-inflammatory effects of antidepressants [43, 44], which may mitigate the hypothesized influences of anti-inflammatory agents on the risk of breast cancer [45]. In addition, the long-term use of antidepressants may also be beneficial in stabilizing mood patterns, and we hypothesize that patients who receive regular treatment for their mental illness may have a healthier lifestyle, which could reduce environmental risk factors for cancer. Furthermore, citalopram, sertraline, and fluvoxamine are relatively weaker inhibitors of CYP2D6 compared to stronger inhibitors such as paroxetine or fluoxetine [46]. Previous studies have suggested that CYP2D6 inhibitors such as paroxetine may impair the conversion of tamoxifen into its active form and hinder its efficacy in treating breast cancer [47, 48]. Taken together, these findings suggest the advantages of citalopram, sertraline, and fluvoxamine in either reducing the risk of breast cancer or the effect on tamoxifen. In addition to biological etiologies, the long-term use of mood stabilizers or antidepressants may also have therapeutic effects. Taking psychotropic agents regularly may improve mental health, and consequently patients may experience less stress and pay more attention to their physical health. Therefore, a balanced mental state (achieved through drug treatment) may have a positive effect on preventing the development of cancer.
Association between the use of antipsychotics and risk of breast cancer
The long-term use (cDDD ≥ 365) of SGAs (OR: 0.8) and FGAs (OR: 0.72) showed a trend towards a decreased risk of breast cancer, while the short-term use (30–179) of SGAs overall, olanzapine, risperidone, and chlorpromazine was significantly associated with a decreased risk of breast cancer. These results differ from previous literature [10, 12] that reported an association between an increased risk of breast cancer and prolactin-related antipsychotics such as risperidone. Several factors may contribute to this discrepancy. First, the association between prolactin secretion and the incidence of breast cancer remains controversial [15]. While some studies have suggested that prolactin stimulates cellular proliferation, differentiation, and angiogenesis of breast cancer [13], other studies have not supported this hypothesis. There is also evidence suggesting that prolactin may have a protective effect and suppress breast cancer cell growth. A preclinical study demonstrated that prolactin could suppress cellular growth or metastasis of breast cancer [14], and another study indicated that the 16-kDa prolactin isoform, a prolactin fragment, had anti-angiogenic effects in in vivo experiments [49]. These findings suggest the potentially biological mechanism by which prolactin-related antipsychotics may reduce the risk of breast cancer. Second, treatment with antipsychotics may improve the symptoms of BD or MDD, which could explain our findings. An observational study of individuals with SMI reported that poorer insight and awareness of the illness were significantly associated with greater disease severity [50]. Previous reviews have shown that SMI is associated with the under-utilization of preventive services and less regular mammography [4, 5, 51]. Therefore, we hypothesize that antipsychotics can help to reduce the severity of illness, leading to improved awareness of self-health and early identification of breast masses during the precancer stage. Third, differences in the study populations may also play a role. Previous studies have recruited patients with schizophrenia [10], while we recruited patients with BD or MDD. Exposure to antipsychotics differs between schizophrenia and mood disorders. For instance, the recommended dose of risperidone for the adjunctive treatment of MDD is 1 to 3 mg per day [52], while the recommended dose for BD is 2 to 4 mg per day [53]. However, the recommended dose of risperidone for schizophrenia is 3 to 6 mg per day [54], indicating a higher cumulative daily dose of antipsychotics compared to BD or MDD. Further studies are needed to clarify the complicated etiologies behind the effect of different mental illnesses. Surprisingly, we found that ziprasidone increased the risk of breast cancer. To the best of our knowledge, no other study has investigated the association between ziprasidone and increased risk of breast cancer, although one preclinical study demonstrated that ziprasidone could suppress pancreatic adenocarcinoma cell proliferation, indicating a potentially anti-cancer effect [55]. However, the relatively wide confidence interval (1.47–15.07) suggests that this result may be confounded by statistical power or chance.
Limitations
There are several limitations to the current study. First, some ORs could not be estimated due to differences in the logistic regression model, including fluoxetine. Second, due to limitations imposed by the institutional review board, the frequency of mammography could not be identified, however this may have affected the risk of breast cancer. Third, the CCI can only evaluate the potential effect of medical comorbidities, however it is difficult to ascertain to what extent these comorbidities affect breast cancer. Fourth, the NHIRD only includes residents in Taiwan, which may limit the generalizability of our findings to a more global context. Fifth, we combined patients with BD and patients with MDD in the analysis regarding the required sample size. Finally, although we adjusted for multiple factors related to the risk of breast cancer, residual confounding factors may still exist, which may have affected the associations between psychotropic drugs and the risk of breast cancer in our study.
Conclusions
Our results showed that treatment with several mood stabilizers (valproic acid), antidepressants (citalopram, sertraline, and fluvoxamine), or antipsychotics (chlorpromazine, olanzapine, or risperidone) was associated with a decreased risk of breast cancer in female patients with BD or MDD. These findings provide valuable information for clinicians when discussing psychotropic drug options with patients who have MDD or BD, particularly those with other risk factors for breast cancer. However, the use of antipsychotics for patients with BD or MDD still needs to be decided based on the patient’s psychiatric symptoms and physical condition. Further neurobiological investigations are needed to better understand the underlying etiologies behind the association between the use of psychotropic drugs and breast cancer.
Data availability
Anonymized data, as described in this manuscript, will be shared upon request from any qualified investigator by the corresponding author (Dr. Mu-Hong Chen, email: kremer7119@gmail.com).
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-386
Dragioti E, Radua J, Solmi M, Gosling CJ, Oliver D, Lascialfari F, Ahmed M, Cortese S, Estrade A, Arrondo G, Gouva M, Fornaro M, Batiridou A, Dimou K, Tsartsalis D, Carvalho AF, Shin JI, Berk M, Stringhini S, Correll CU, Fusar-Poli P (2023) Impact of mental disorders on clinical outcomes of physical diseases: an umbrella review assessing population attributable fraction and generalized impact fraction. World Psychiatry 22:86–104
Solmi M, Firth J, Miola A, Fornaro M, Frison E, Fusar-Poli P, Dragioti E, Shin JI, Carvalho AF, Stubbs B, Koyanagi A, Kisely S, Correll CU (2020) Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. Lancet Psychiatry 7:52–63
Levinson Miller C, Druss BG, Dombrowski EA, Rosenheck RA (2003) Barriers to primary medical care among patients at a community mental health center. Psychiatr Serv 54:1158–1160
Owen C, Jessie D, De Vries RM (2002) Barriers to cancer screening amongst women with mental health problems. Health Care Women Int 23:561–566
Ren Q, Luo F, Ge S, Chen P (2023) Major depression disorder may causally associate with the increased breast cancer risk: Evidence from two-sample mendelian randomization analyses. Cancer Med 12:1984–1996
Cui Y, Lu W, Shao T, Zhuo Z, Wang Y, Zhang W (2023) Severe mental illness and the risk of breast cancer: a two-sample, two-step multivariable Mendelian randomization study. PLoS ONE 18:e0291006
Chen VC, Liao YT, Yeh DC, Tseng HC, Stewart R, Lee CT (2016) Relationship between antidepressant prescription and breast cancer: a population based study in Taiwan. Psychooncology 25:803–807
Chubak J, Bowles EJ, Yu O, Buist DS, Fujii M, Boudreau DM (2016) Breast cancer recurrence in relation to antidepressant use. Cancer Causes Control 27:125–136
Taipale H, Solmi M, Lahteenvuo M, Tanskanen A, Correll CU, Tiihonen J (2021) Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry 8:883–891
Wawruszak A, Halasa M, Okon E, Kukula-Koch W, Stepulak A (2021) Valproic acid and breast cancer: state of the art in 2021. Cancers (Basel). 13:3409
Rahman T, Sahrmann JM, Olsen MA, Nickel KB, Miller JP, Ma C, Grucza RA (2022) Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol 42:7–16
Clevenger CV, Furth PA, Hankinson SE, Schuler LA (2003) The role of prolactin in mammary carcinoma. Endocr Rev 24:1–27
Nouhi Z, Chughtai N, Hartley S, Cocolakis E, Lebrun JJ, Ali S (2006) Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res 66:1824–1832
De Hert M, Peuskens J, Sabbe T, Mitchell AJ, Stubbs B, Neven P, Wildiers H, Detraux J (2016) Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand 133:5–22
Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, Wahlbeck K, Tiihonen J (2010) Incidence of cancer and antidepressant medication: record linkage study. Int J Cancer 126:285–296
Kato I, Zeleniuch-Jacquotte A, Toniolo PG, Akhmedkhanov A, Koenig K, Shore RE (2000) Psychotropic medication use and risk of hormone-related cancers: the New York University Women’s Health Study. J Public Health Med 22:155–160
Meng Q, Chen X, Sun L, Zhao C, Sui G, Cai L (2011) Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem 348:165–171
Pellegrino M, Rizza P, Nigro A, Ceraldi R, Ricci E, Perrotta I, Aquila S, Lanzino M, Ando S, Morelli C, Sisci D (2018) FoxO3a mediates the inhibitory effects of the antiepileptic drug lamotrigine on breast cancer growth. Mol Cancer Res 16:923–934
Huang JS, Yang FC, Chien WC, Yeh TC, Chung CH, Tsai CK, Tsai SJ, Yang SS, Tzeng NS, Chen MH, Liang CS (2021) Risk of substance use disorder and its associations with comorbidities and psychotropic agents in patients with autism. JAMA Pediatr 175:e205371
Liang CS, Bai YM, Hsu JW, Huang KL, Ko NY, Chu HT, Yeh TC, Tsai SJ, Chen TJ, Chen MH (2020) The risk of sexually transmitted infections following first-episode schizophrenia among adolescents and young adults: a cohort study of 220 545 subjects. Schizophr Bull 46:795–803
Chen CJ, Lin LH (1997) Use of national health insurance database in academic research: experiences from analysis of major disease certification profile. Chin J Publ Health 16:513–521
Chen MH, Hsu JW, Huang KL, Su TP, Li CT, Lin WC, Tsai SJ, Cheng CM, Chang WH, Pan TL, Chen TJ, Bai YM (2019) Risk and coaggregation of major psychiatric disorders among first-degree relatives of patients with bipolar disorder: a nationwide population-based study. Psychol Med 49:2397–2404
Chen MH, Lan WH, Hsu JW, Huang KL, Su TP, Li CT, Lin WC, Tsai CF, Tsai SJ, Lee YC, Chen YS, Pan TL, Chang WH, Chen TJ, Bai YM (2016) Risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: a nationwide longitudinal study. Diabetes Care 39:788–793
Cheng CM, Chang WH, Chen MH, Tsai CF, Su TP, Li CT, Tsai SJ, Hsu JW, Huang KL, Lin WC, Chen TJ, Bai YM (2018) Co-aggregation of major psychiatric disorders in individuals with first-degree relatives with schizophrenia: a nationwide population-based study. Mol Psychiatry 23:1756–1763
Huang MH, Cheng CM, Tsai SJ, Bai YM, Li CT, Lin WC, Su TP, Chen TJ, Chen MH (2020) Familial coaggregation of major psychiatric disorders among first-degree relatives of patients with obsessive-compulsive disorder: a nationwide study. Psychol Med. 51:680–687
Katki HA, Berndt SI, Machiela MJ, Stewart DR, Garcia-Closas M, Kim J, Shi J, Yu K, Rothman N (2023) Increase in power by obtaining 10 or more controls per case when type-1 error is small in large-scale association studies. BMC Med Res Methodol 23:153
Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS (2006) Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Management (Chin) 4:1–22
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Lofling L, Sundstrom A, Kieler H, Bahmanyar S, Linder M (2019) Exposure to antimuscarinic medications for treatment of overactive bladder and risk of lung cancer and colon cancer. Clin Epidemiol 11:133–143
Lin TC, Lee CH, Yang CY, Yang YH, Lin SJ (2014) Incidence and risk of venous thromboembolism among Taiwan osteoporotic fracture population under osteoporosis pharmacological treatments. J Clin Endocrinol Metab 99:1599–1607
Tu TH, Huang KL, Bai YM, Hsu JW, Su TP, Li CT, Tsai SJ, Lin WC, Chen TJ, Chen MH (2019) Exposure to second-generation antipsychotics and risk of type 2 diabetes mellitus in adolescents and young adults: a nationwide study in Taiwan. J Clin Psychiatr. https://doi.org/10.4088/JCP.18m12284
Liu HP, Wei JC, Yip HT, Yeh MH (2021) Association of insomnia, depressive disorders, and mood disorders as risk factors with breast cancer: a nationwide population-based cohort study of 232,108 women in taiwan. Front Oncol 11:757626
Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, Leung J, Ravindran AV, Chen WQ, Qiao YL, Shi J, Lu L, Bao YP (2020) Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry 25:1487–1499
Lin GM, Chen YJ, Kuo DJ, Jaiteh LE, Wu YC, Lo TS, Li YH (2013) Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997–2009. Schizophr Bull 39:407–416
Li Z, Yang L, Zhang S, Song J, Sun H, Shan C, Wang D, Liu S (2021) Valproic acid suppresses breast cancer cell growth through triggering pyruvate kinase M2 isoform mediated Warburg effect. Cell Transplant 30:9636897211027524
Heers H, Stanislaw J, Harrelson J, Lee MW (2018) Valproic acid as an adjunctive therapeutic agent for the treatment of breast cancer. Eur J Pharmacol 835:61–74
Brown SB, Hankinson SE, Arcaro KF, Qian J, Reeves KW (2016) Depression, antidepressant use, and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 25:158–164
Emiliano AB, Fudge JL (2004) From galactorrhea to osteopenia: rethinking serotonin-prolactin interactions. Neuropsychopharmacology 29:833–846
Madhusoodanan S, Parida S, Jimenez C (2010) Hyperprolactinemia associated with psychotropics–a review. Hum Psychopharmacol 25:281–297
Reeves KW, Okereke OI, Qian J, Tamimi RM, Eliassen AH, Hankinson SE (2018) Depression, antidepressant use, and breast cancer risk in pre- and postmenopausal women: a prospective cohort study. Cancer Epidemiol Biomarkers Prev 27:306–314
Reeves KW, Okereke OI, Qian J, Tworoger SS, Rice MS, Hankinson SE (2016) Antidepressant use and circulating prolactin levels. Cancer Causes Control 27:853–861
Kenis G, Maes M (2002) Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol 5:401–412
Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM (2009) Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther 86:527–532
Ma S, Guo C, Sun C, Han T, Zhang H, Qu G, Jiang Y, Zhou Q, Sun Y (2021) Aspirin use and risk of breast cancer: a meta-analysis of observational studies from 1989 to 2019. Clin Breast Cancer 21:552–565
Hemeryck A, Belpaire FM (2002) Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drug Metab 3:13–37
Desmarais JE, Looper KJ (2009) Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 70:1688–1697
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Faupel-Badger JM, Ginsburg E, Fleming JM, Susser L, Doucet T, Vonderhaar BK (2010) 16 kDa prolactin reduces angiogenesis, but not growth of human breast cancer tumors in vivo. Horm Cancer 1:71–79
Rozalski V, McKeegan GM (2019) Insight and symptom severity in an inpatient psychiatric sample. Psychiatr Q 90:339–350
Mitchell AJ, Pereira IE, Yadegarfar M, Pepereke S, Mugadza V, Stubbs B (2014) Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry 205:428–435
Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Muller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R, Group CDW (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological Treatments. Can J Psychiatry. 61:540–560
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, Sharma V, Goldstein BI, Rej S, Beaulieu S, Alda M, MacQueen G, Milev RV, Ravindran A, O’Donovan C, McIntosh D, Lam RW, Vazquez G, Kapczinski F, McIntyre RS, Kozicky J, Kanba S, Lafer B, Suppes T, Calabrese JR, Vieta E, Malhi G, Post RM, Berk M (2018) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 20:97–170
Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M (2017) Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry 62:604–616
Yang Y, Zheng M, Han F, Shang L, Li M, Gu X, Li H, Chen L (2022) Ziprasidone suppresses pancreatic adenocarcinoma cell proliferation by targeting GOT1 to trigger glutamine metabolism reprogramming. J Mol Med (Berl) 100:599–612
Acknowledgements
The authors thank Mr I-Fan Hu, MA (Courtauld Institute of Art, University of London; National Taiwan University) for his friendship and support. Mr Hu declares no conflicts of interest.
Funding
Open Access funding enabled and organized by National Yang Ming Chiao Tung University. The study was supported by grants from Taipei Veterans General Hospital (V106B-020, V107B-010, V107C-181, V108B-012, V110C-025, V110B-002), Yen Tjing Ling Medical Foundation (CI-109-21, CI-109-22, CI-110-30) and Ministry of Science and Technology, Taiwan (107-2314-B-075-063-MY3, 108-2314-B-075 -037, 110-2314-B-075-026, 110-2314-B-075-024-MY3). The funding sources had no role in any process of our study.
Author information
Authors and Affiliations
Contributions
Drs MHC and CSL: designed the study. Drs DJL, MHC, CSL: wrote the draft; Drs DJL, SJT, and TJC: performed the literature review and revised the manuscript; Dr MHC: performed the statistical analysis; all authors reviewed the final manuscript and agreed with its publication.
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest.
Ethical approval
This study protocol was reviewed and accepted by the Institutional Review Board of Taipei Veterans General Hospital (approval number: TPEVGH-IRB-2018–07-016AC). The requirement for patient consent was waived because the data used in this study were anonymized and derived wholly from a sizeable national database. All authors have no financial relationships relevant to this article to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, DJ., Tsai, SJ., Chen, TJ. et al. Exposure to psychotropic drugs and breast cancer risk in patients with bipolar disorder and major depressive disorder: a nested case–control study. Eur Arch Psychiatry Clin Neurosci (2024). https://doi.org/10.1007/s00406-024-01798-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-024-01798-9