Abstract
Depression is common in attention-deficit/hyperactivity disorder (ADHD), but preventive behavioural interventions are lacking. This randomised controlled, pilot phase-IIa trial aimed to study a physical exercise intervention (EI) and bright light therapy (BLT)—both implemented and monitored in an individual, naturalistic setting via a mobile health (m-health) system—for feasibility of trial design and interventions, and to estimate their effects on depressive symptoms in young people with ADHD. Two hundred seven participants aged 14–45 years were randomised to 10-week add-on intervention of either BLT (10,000 lx; daily 30-min sessions) (n = 70), EI (aerobic and muscle-strengthening activities 3 days/ week) (n = 69), or treatment-as-usual (TAU) (n = 68), of whom 165 (80%) were retained (BLT: n = 54; EI: n = 52; TAU: n = 59). Intervention adherence (i.e. ≥ 80% completed sessions) was very low for both BLT (n = 13, 22%) and EI (n = 4, 7%). Usability of the m-health system to conduct interventions was limited as indicated by objective and subjective data. Safety was high and comparable between groups. Changes in depressive symptoms (assessed via observer-blind ratings, Inventory of Depressive Symptomatology) between baseline and end of intervention were small (BLT: −0.124 [95% CI: −2.219, 1.971], EI: −2.646 [95% CI: −4.777, −0.515], TAU: −1.428 [95% CI: −3.381, 0.526]) with no group differences [F(2,153) = 1.45, p = 0.2384]. These findings suggest that the m-health approach did not achieve feasibility of EI and BLT in young people with ADHD. Prior to designing efficacy studies, strategies how to achieve high intervention adherence should be specifically investigated in this patient group.
Trial registration
ClinicalTrials.gov, NCT03371810, 13 December 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental condition with onset in childhood and a high rate of persistence into adulthood [1]. ADHD is associated with co-occurring mental [1] and somatic conditions [2] that add to individual disease burden [2,3,4]. Depression [5] and obesity [2, 6] are amongst the most common conditions, with increasing prevalence rates during transition from childhood into adulthood [2,3,4, 6, 7]. There is little evidence that first-line pharmacological and non-pharmacological ADHD interventions may prevent especially depression [8]. Non-adherence to medication increases during adolescence [9], further complicating effective prevention of co-occurring conditions during this particularly risky developmental phase. A wider range of non-pharmacological options that directly target known pathophysiological mechanisms of ADHD, depression and obesity is, thus, necessary.
Physical exercise attenuates the health risk of obesity [10] and is implemented in programmes to prevent and reduce obesity [11] and depression in young people [12,13,14,15]. Physical exercise is thought to directly modulate dysregulation of the dopamine system—a key pathophysiological mechanism of ADHD which also plays a role in mood disorders and obesity [16]. Following the idea of a shared disturbance of the circadian system which may link ADHD to depression [17] and obesity [18], also bright light therapy (BLT) may prevent both conditions. With morning light administration, phase delays in the sleep/wake cycle characteristic of ADHD [17] can be shifted to an earlier time. Stabilising circadian rhythms through BLT is effective in reducing depressive symptoms [19] and accumulating evidence suggests its efficacy in eating disorders and obesity [20].
To prevent increase in depressive symptoms and obesity in young people with ADHD, we developed two manualised 10-week interventions of physical exercise (EI) and BLT, both combined with a novel mobile health (m-health) system to support participants’ engagement. m-Health approaches have gained considerable popularity to promote lifestyle changes in adults [21] and adolescents [22]. There is limited evidence of their feasibility and efficacy as a tool to implement, monitor and reinforce behavioural changes in psychiatric populations, specifically young people with ADHD [23]. The m-health system included a smartphone app to deliver and prompt BLT and EI in an individual, naturalistic setting. Importantly, the app allowed online monitoring of participants’ intervention adherence, which is a critical factor for feasibility but is often not sufficiently addressed in randomised controlled trials (RCTs) [12, 24, 25]. In addition, the m-health system included a wrist-worn mobile sensor to record online physical activity and light exposure. A daily feedback mechanism provided individual reward summaries based on the data recorded online to increase engagement and motivation.
This pilot phase-IIa RCT on BLT versus EI, both in combination with m-health-based monitoring and reinforcement, addressed feasibility of trial design and interventions in adolescents and young adults with ADHD in terms of (i) recruitment and retention, (ii) data collection methods, (iii) usability of the m-health system, (iv) intervention adherence, (v) intervention integrity, and (vi) safety of interventions [26]. To inform a future definitive RCT, we established effect sizes of the primary efficacy outcome for depressive symptoms and secondary efficacy outcomes for obesity and ADHD symptoms [27].
Methods
Study design
This multicentre study is a pilot phase-IIa trial [27,28,29] with a prospective, randomised controlled, observer-blind, parallel-group design, comparing m-health based BLT and EI with treatment-as-usual (TAU) in participants with ADHD. Reporting was guided by the Consolidated Standards of Reporting Trials extension to randomised pilot and feasibility trials guidelines [26]. The study was conducted at four European centres (Goethe University Hospital Frankfurt, Germany; King’s College London, UK; Radboud University Medical Centre, Nijmegen, The Netherlands; Vall d’Hebron Research Institute, Barcelona, Spain). The study protocol [30] complied with the declaration of Helsinki (revision) and was approved by the institutional review boards of all centres [see Supplementary Information (SI) 1]. All participants provided written informed consent before their participation in the trial. For participants aged 14–17 years, written informed consent was also obtained from legal guardians.
Participants
Eligible participants (aged 14–45 years) were recruited from clinical departments that collaborated with or were part of the participating centres, and by public announcements. All participants met DSM-5 criteria for a lifetime history of childhood-onset ADHD as well as current ADHD criteria established by psychiatric expert assessment based on structured clinical interviews. Details on procedures, inclusion, and exclusion criteria are reported in the online supporting information (SI2 and Supplementary Table 1).
Interventions
See SI3 for detailed descriptions of the 10-week interventions and the m-health system (Supplementary Fig. 1 and 2). Briefly, BLT consisted of a daily (except Sunday) individualised, home-based 30-min exposure of white light provided by a 10,000 lx light box that supplied broadband, UV-filtered light (Philips EnergyLight HF 3419). The exact time of day of implementation (either in the morning [6–8 am] or in the evening [6–8 pm]) was determined by the chronotype of each participant.
Following the internationally accepted physical activity guidelines, EI included aerobic exercise of moderate-to-vigorous intensity and muscle-strengthening activities on 3 days a week. Based on participant’s baseline cardiorespiratory fitness, they were assigned to one of three programmes of light, moderate or high intensity. Strengthening exercises were presented in the form of video sessions on a smartphone and were executed whilst watching the videos. Aerobic activities included, for example, running, brisk walking or bicycling, and were individually chosen by participants.
Both interventions were individually incorporated into participants’ daily routines. Instruction, monitoring and feedback were realised with the m-health system comprising a smartphone (Motorola Moto G3) equipped with the m-health app (movisensXS software, movisens GmbH), and a wrist-worn light and activity sensor (LightMove 3, movisens GmbH).
TAU included stable psychopharmacotherapy for ADHD, stable medication for chronic medical conditions not interfering with interventions, individual- or group-based psychotherapy, or family support. BLT and EI were provided as add-on therapies to TAU.
Randomisation and masking
Eligible participants were successively randomised in a 1:1:1 ratio to one of the three groups using a centralised web-based tool. Randomisation was done successively during the trial (April 2017- March 2020) in blocks with fixed length and stratified for each centre. Observers rating the severity of depressive (Inventory of Depressive Symptomatology, IDS-C30) and ADHD symptoms (ADHD Rating Scale) were masked to intervention allocation at all visits. Adherence to randomisation and masking procedures was monitored by an independent clinical on-site monitor throughout the trial (SI4).
Procedures
Following screening (at visit T1) and baseline assessments (at visit T2 and with the m-health system on four days at home between T1 and T2), participants were randomised and then introduced to the respective intervention/TAU by trained study staff at T2. The devices for BLT and EI and a user’s guide were handed over. The next day, participants started either BLT or EI in addition to TAU. The control group continued with TAU. Follow-up visits occurred after 5 weeks (mid-intervention assessment, T3), after 10 weeks (end of intervention assessment, T4 including an assessment with the m-health system on four days at home), and 12–14 weeks after T4 (follow-up assessment, T5). After start of the COVID-19 pandemic, follow-up assessments were obtained via phone (SI1). Standardised motivational interviews were conducted at T2 and T3 in case of low self-reported intervention adherence (i.e. < 80% completed sessions).
Outcome measures
Feasibility endpoints
Feasibility outcomes in terms of the trial design were screening, recruitment, and retention rates, duration of recruitment, and number of protocol violations. To determine the feasibility of data collection methods, number of missing data was reported for primary and secondary efficacy outcomes at each visit in the randomised population. We also reported the number of missing sensor data sets at baseline and during 10 weeks of intervention. For ratings of depressive and ADHD symptoms, we determined the percentage of observer-blind ratings at each visit and calculated interrater reliability. As a further feasibility assessment, we included participants’ adherence to interventions (BLT/ EI) based on the data recorded online with the m-health app. Specifically, we calculated the percentage of conducted sessions, the total duration of conducted sessions during 10 weeks of intervention and the mean session duration. In addition, we monitored via the sensor the mean light exposure (in lux) and physical activity (number of steps, movement acceleration) per day during 10 weeks of BLT/ EI. Participants’ adherence to interventions was also assessed retrospectively via interview at T3. Participants rated the usability of the m-health app for conducting BLT and EI using the System Usability Scale (SUS). We also monitored the wearing time of the sensor. At each visit, we recorded any change in prescribed concomitant medication and psychosocial treatment. Participants’ self-reports on continuation with BLT/ EI after T4 were included to determine acceptability of interventions. Treatment integrity was assessed via study staffs’ adherence to delivering the prescribed intervention protocol (see SI5).
All serious adverse events (SAEs) and adverse events (AEs) that caused physical or psychological harm were recorded via self-reports at each visit (SI6 and Supplementary Table 3 and 4).
Efficacy endpoints
The primary efficacy outcome was the change in the IDS-C30 total score between T2 and T4. Changes in the IDS-C30 total score between T2 and T5 and changes in self-reported depressive symptoms (Beck Depression Inventory, BDI) between T2 and the follow-up visits (T4, T5) were secondary outcomes. In terms of obesity, we included changes in body mass index, body fat percentage, waist circumference, and waist-to-hip ratio between baseline and follow-up visits (T4, T5). Furthermore, we assessed changes in ADHD symptoms (ADHD Rating Scale) between baseline and follow-up visits (see SI7).
Statistical analysis
The sample size was determined to detect a clinically relevant medium effect size (d = 0.5) for the primary efficacy endpoint between at least one of the two interventions compared to TAU with a two-sample t test. As this was a pilot study, the sample size was planned at the liberal significance level of α = 0.10 (two-sided) and a power of 1-β = 80%, resulting in a sample size of 153 (n = 51 per group). A target of 219 randomisations was planned to compensate for an expected drop-out rate of 30%. For details on statistical analyses, see SI8.
Feasibility endpoints
We summarised feasibility outcomes using descriptive statistics including the mean (SD) for continuous data and frequencies and percentages for categorical variables. Feasibility endpoints in terms of the trial design and data collection methods were analysed in the set of all randomised participants. Participants’ adherence and acceptability of interventions, the usability and acceptability of the m-health system, and treatment integrity were evaluated in the set of participants who started the respective intervention after randomisation. Mean physical activity and light exposure were calculated for participants who wore the sensor for at least 8 h during time awake per day. Frequencies of AEs and SAEs were evaluated in the set of all randomised participants (see SI6). Additional analyses were conducted to explore predictors of participants’ adherence to interventions (BLT/ EI) by means of multivariable linear regression models. Exploratory analyses also included a cluster analysis to identify different subgroups of participants based on baseline demographic and clinical variables, which resulted in three clusters of ADHD individuals characterised by differential IQ, education of parents, age, BMI, and ADHD symptom distribution. Intervention adherence was also explored in these subgroups (for details see SI9).
Primary efficacy endpoint
Changes in the IDS-C30 between T2 and T4 were analysed as randomised in the modified intention-to-treat (mITT) population (see SI8). The mITT set consisted of all participants who were randomised, with IDS-C30 total scores at T2 and at least at one follow-up visit (either T3 or T4). A closed testing procedure was applied to control the overall type I error rate at 0.05. A mixed model for repeated measures (MMRM) with the restricted maximum likelihood estimation method was used to investigate the treatment effect with respect to all three groups. Within-patient errors were modelled with an unstructured covariance structure. Two group comparisons were provided by pre-defined contrasts. Baseline IDS-C30, age, IQ, sex, treatment, centre, visit and treatment-by-visit interaction were included as covariates. The related two-sided 95% confidence intervals for the intervention group differences were calculated. The above-described confirmatory approach controlling the type I error rate at 0.05 was pursued to enable a proof of efficacy already in this pilot study. If the effect size is d = 0.5 as assumed for sample size calculation, the power to reject the null hypothesis of no difference in the primary endpoint comparing one novel intervention to TAU is only 70% (instead of 80%) as planning was performed at the more liberal level of 0.10.
Additional analyses of the primary efficacy endpoint
Sensitivity analyses were conducted for different populations (a per-protocol population including participants without major protocol violations, complete case analysis) and applying different imputation techniques for missing values (SI8). Intervention effects on the primary efficacy endpoint were also compared between the different subgroups identified by the exploratory cluster analysis (SI9).
Secondary efficacy endpoints
Changes in secondary efficacy outcomes were analysed as randomised in the mITT population. The change regarding IDS-C30 between T2 and T5 was compared between intervention groups using the same MMRM model as in the primary analysis. This analysis was based on the follow-up data up to visit T5. Group comparisons regarding changes of all other secondary efficacy endpoints were conducted using the same MMRM models as described for the analysis of IDS-C30 (using the respective endpoint at baseline as a covariate instead of baseline IDS-C30). Baseline characteristics were summarised descriptively in the set of all randomised participants and the mITT population.
Results
Feasibility of trial design
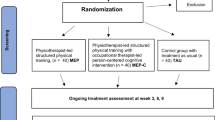
Between April 4, 2017 and March 31, 2020, 553 participants were screened (Supplementary Table 5), of whom 207 participants (mean age 25.8 [SD = 8.0]; range 14–44; 55.1% females [n = 114]) were eligible and randomly assigned to receive either BLT (n = 70), EI (n = 69), or TAU (n = 68) (Fig. 1). Follow-up assessments were done between May 16, 2017 and August 31, 2020. The mITT set included 174 (84%) of the randomised participants (BLT, n = 59, EI, n = 54, TAU, n = 61). The target sample size (n = 51) of the original power analysis was reached in each group for the primary efficacy analysis. Baseline characteristics are summarised in Table 1 for all randomised participants (see Supplementary Table 6 for the mITT set).
The recruitment rate was 95% of the target sample size. Still, there were many participants who were assessed for eligibility but not enrolled (n = 346 of 553 screened individuals; 63%). Therefore, inclusion criteria were adapted (inclusion of participants up to 45 years old), and the study timeline was extended by 5 months. Two participants (1%) did not complete the T2 visit, and therefore did not receive their intervention after randomisation. At T3, 178 participants (total: 86%, BLT: 87%, EI: 80%, TAU: 91%) were retained, and 165 participants (total: 80%, BLT: 77%, EI: 75%, TAU: 87%) were retained at T4. Retention at follow-up (T5) was lower (total: n = 147, 71%, BLT: 70%, EI: 68%, TAU: 75%). Premature study termination occurred mostly because participants either declined further participation in the study (n = 20, 33%) or were lost to follow-up (n = 21, 35%). Five participants (1 adolescent, 4 adults, 8%) were admitted to psychiatric inpatient ward, and thus could not further participate. None of these admissions was due to study intervention. Protocol violations (i.e. deviation of T4 visit more than ± 7 days from the planned visit) were documented in 72% of participants (n = 150). These participants were excluded from the per-protocol analysis (Fig. 1).
At T4, IDS-C30 ratings were not available for 42 drop-outs and for additional 3 participants who did not take part in the interview (see Supplementary Table 7 and 8 for details on missing data). Across visits, the vast majority of IDS-C30 ratings was done observer-blind (T2: 97%; T3: 99%, T4: 96%, T5: 97%), and interrater reliability was good to excellent [ICC(2,1) = 0.93 (95% CI: 0.87, 0.98), see SI4 and Supplementary Table 2]. ADHD ratings were also done observer-blind (T2: 95%; T3: 97%, T4: 96%, T5: 95%) with moderate to good interrater reliability [ICC(2,1) = 0.80 (95% CI: 0.63, 0.93)].
Feasibility of interventions
Feasibility of the m-health system: At baseline, sensor data were not available for 38 (18%) randomised participants. The wearing time of the sensor per day was high and comparable across groups (mean percentage, BLT, 73.9% [SD = 22.8]; EI, 77.5% [SD = 24.7]; TAU, 72.2% [SD = 28.7]). During intervention, sensor data sets were not available for 15 (11%) participants who received an intervention. The wearing time of the sensor dropped to 30.1% in the BLT group and to 41.4% in the EI group (Supplementary Table 9 and 10). Participants rated the m-health app as acceptable for conducting BLT (SUS, mean score 76.1), but less so for EI (mean score 65.5) (Supplementary Table 11).
Feasibility of BLT and EI
In terms of intervention adherence, participants assigned to BLT completed on average 53% (SD = 0.4) of the prescribed 60 sessions, based on the app data (missing data sets, n = 9, 13%). This corresponds to a mean total duration of 960 min (SD = 617.5) of BLT over 10 weeks of intervention. Adherence to BLT defined as 80% or higher of completed sessions was objectively observed only for 13 participants (22%) (Fig. 2, Supplementary Table 12). In contrast, at T3, 78.9% of participants subjectively reported adherence to BLT (i.e. ≥ 80% completed sessions) over the first 5 weeks of intervention. None of the participants continued with BLT after T4 as indicated by self-reports. Mean light exposure per day was similar for both intervention groups during the intervention period (Supplementary Table 13). Exploratory regression analyses revealed that higher adherence to BLT was significantly associated with lower scores on the ADHD Rating Scale (reduced model, estimate = −0.9873, t = −2.47 p = 0.016). Participants’ adherence to BLT did not significantly differ between the three subgroups of participants identified by exploratory cluster analysis (SI9).
Participants assigned to EI completed on average 28% (SD = 28.3) of the prescribed sessions (missing data sets, n = 9, 13%) with only four participants (6.8%) objectively showing intervention adherence (≥80% completed sessions) (Fig. 2, Supplementary Table 14). On average, participants completed 31.2% (SD = 28.4) of aerobic exercise sessions [mean total duration: 354.8 min (SD = 331.6)] and 23.9% (SD = 30.3) of strengthening sessions. At T3, 50.0% of participants subjectively reported adherence to intervention over the first 5 weeks. Twenty-three participants reported that they had continued with physical exercises after T4 (mean number of sessions per month: 8.9 [SD = 4.2]). Descriptively, physical activity (mean number of steps and movement acceleration) was higher for participants assigned to EI than BLT during the intervention period (Supplementary Table 13). Adherence to EI was strongly associated with higher age (full model, estimate = 0.1587, t = 4.35 p < 0.0001), but not any other baseline demographic or clinical variables. The subgroup of participants (cluster 3) who differed predominantly in terms of age from the other two clusters showed significantly better adherence to EI compared to the younger subgroups (p = 0.002; see SI9).
Self-reported adherence to TAU was high (96.6%) as subjectively assessed at T3 in the control group. During study participation, changes in type of concomitant medication occurred in 39 (19%) participants who started the intervention/ TAU (BLT: n = 15, 22%, EI: n = 12, 18%, TAU: n = 12, 18%) whilst psychosocial treatment was stable (Supplementary Table 15).
Study staff reported that all participants were introduced to the respective intervention and the m-health system according to the pertinent standard operating procedures (SOPs). The majority of participants who reported no adherence to intervention/ TAU at T3 received motivational interviewing (BLT, 70%; EI, 78%; TAU, 25%).
Safety: AEs were reported in 99 (48%) participants with similar frequencies across groups [TAU: n = 29 (43%), BLT: n = 39 (56%), EI: n = 31 (45%)]. Causality with intervention was mostly considered unrelated or unlikely (n = 90). Intervention-related AEs (i.e. headaches) occurred in two participants from the BLT group, which led to premature study termination in one participant. SAEs were reported in six participants [TAU: n = 2 (2.9%), BLT: n = 1 (1.4%), EI: n = 3 (4.3%)] with no SAE deemed to be caused by study intervention (Supplementary Table 3 and 4).
Efficacy of interventions
In terms of the primary efficacy endpoint, changes in IDS-C30 total score between baseline and T4 were small (see Table 2, Supplementary Fig. 3 and 4) (BLT: −0.124 [95% CI: −2.219, 1.971], EI: −2.646 [95% CI: −4.777, −0.515], TAU: −1.428 [95% CI: −3.381, 0.526]) and not different between groups [F(2,153) = 1.45, p = 0.2384]. For both interventions, intervention adherence had no effect on changes in IDS-C30 total score at T4. Applying last-observation-carried-forward as an imputation technique for missing values as well as complete case analysis gave very similar results. Per-protocol analyses did not show sufficient power (Supplementary Table 16 and 17). For BLT as well as EI, changes in depressive symptoms from baseline to T4 did not differ between the three clinical subgroups (SI9). With regard to secondary outcomes, there was a reduction from baseline in BMI at T4 in the BLT group whereas mean BMI increased in the TAU and EI groups. The group effect (p = 0.0349) was due to pairwise difference between BLT and TAU (-0.442 [95% CI: −0.776, −0.107]), and was not maintained at T5. Compared to TAU, EI had no effect on BMI at either T4 or T5. For all other secondary outcomes, no intervention effect was observed at either T4 or T5 (Table 2 and Supplementary Table 18).
Discussion
We successfully completed a European multicentre study implementing BLT and EI for prevention of depression in young people with ADHD. The large sample size allowed exploring feasibility of trial design and behavioural interventions prior to a confirmatory trial, which represents a key strength of this pragmatic pilot phase-IIa trial.
Feasibility of trial design
Recruitment of adolescents and young adults was partly challenging, but retention rates were adequate to reach sufficient power for the primary efficacy analysis. Number of missing data was low for the primary efficacy endpoint, observer-blind assessments were done, and reliability between raters from four European sites was good to excellent. Number of missing data was also low for self-reported depressive and observed ADHD symptoms at T4. ADHD ratings were mainly done observer-blind with moderate to good interrater reliability. Body composition parameters were missing more often, and therefore findings regarding obesity need to be interpreted with more caution. Thus, the design and implementation of the trial proved overall feasible concerning recruitment rate, trial retention, masking, and data collection in terms of depression and ADHD outcome measures.
Feasibility of interventions
With regard to interventions, the delivery of BLT and EI by trained study staff also was feasible. BLT and EI were safe with few participants reporting SAEs, which were not related to the interventions. AEs were mostly unrelated to interventions and occurred with similar rates across the three groups. Two BLT participants reported headaches, which are well-described adverse effects.
A key feature of this trial was the implementation of BLT and EI in an individual, naturalistic setting supported by an m-health system. Adequate control of adherence to behavioural interventions is challenging and often neglected in RCTs on exercise, BLT or comparable interventions in clinical populations [12, 24, 25]. This study is one of the few studies to carefully monitor and report on participants’ adherence throughout the intervention. Drop-out rates from intervention arms were comparable to those reported in EI trials for depression and other clinical populations [14] and in RCTs on psychosocial interventions in adults with ADHD [8]. Still, adherence to intervention was far lower. Based on objective m-health monitoring, only around half (BLT) to a quarter (EI) of the prescribed sessions were completed. Similarly, only 22% of BLT and 7% of EI showed intervention adherence. These findings indicate that EI and BLT as implemented in our study were not feasible in young people with ADHD.
To inform future studies, it is crucial to understand possible reasons for low intervention adherence observed in our sample of young people with ADHD, including characteristics and implementation of the interventions themselves, ADHD-related factors, and other demographic and clinical characteristics of participants. To increase motivation, we carefully matched exercise intensity to participants’ current fitness level and timing of BLT to chronotype. Still, integrating the interventions into daily routines required high commitment in terms of time and organisation. In the light of executive and reward function impairments characteristic of ADHD [16], the implementation of individualised BLT and EI in a lowly structured naturalistic setting might have been challenging. In line with this hypothesis, exploratory findings indicate that participants with more severe ADHD symptoms showed less adherence to BLT. Previous research in depressed individuals also suggests that adherence to EI is higher when conducted in inpatient settings or under supervision providing not only more structure but also social support [14]. Also, group settings have motivating effects [12, 14]. In terms of demographic characteristics, exploratory analyses identified age as a strong predictor of intervention adherence in the EI group. Better adherence to EI was also found in the subgroup of participants characterised by higher age indicating that EI as implemented in this study might be more suitable for adults rather than adolescents with ADHD. As most participants had no diagnosis of depression or obesity, participants’ motivation to engage in interventions targeting these possible future co-occurring conditions may have been limited. Importantly, a significant limitation is that the m-health system itself (especially the sensor) was not well accepted by the participants as indicated by objective and subjective data. Wearing time of the sensor was low and acceptability was limited for the more complex EI intervention that required watching videos provided by the app to learn and conduct specific exercises. This might have constrained the validity for providing daily feedback to booster motivation for a behavioural change. When designing further studies on behavioural interventions for individuals with ADHD, co-design methods including the affected individuals should be implemented to overcome the present study’s limitations regarding recruitment and acceptability [31]. Co-design may focus on intervention targets, daily routines and digital mental health technologies and should take ADHD-related factors and age of participants even more into account [32].
Efficacy of interventions
As indicated by the main analyses and additional cluster analyses, intervention adherence had no effect on change of depressive symptoms from baseline to T4, and was overall low. Thus, the efficacy of BLT and EI to prevent depressive symptoms in young people with ADHD remains to be determined. Findings on secondary efficacy outcomes also need to be interpreted with caution given the explorative nature of the analyses. At T4, BLT showed a preventive effect on BMI compared to TAU, but this effect was small with no stability at follow-up, as BLT was not continued. Similarly, no group differences or medium pre–post effects were found for any other secondary efficacy outcome.
Future studies may overcome additional limitations of this study by the following design aspects: first, outcomes were assessed after 10 weeks of intervention and the stability of effects at 3-month follow-up. This time frame might be too short for long-term protective effects [13, 15] as well as for the development or progression of depressive symptoms given. In addition, participants were allowed to receive stable anti-depressive medication (TAU: 13%, BLT: 21%, EI: 20%) and psychosocial treatment (TAU: 18%, BLT: 10%, EI: 19%), which already may have strongly reduced their depressive symptoms, thus making it unlikely that BLT and EI might induce an even stronger reduction. Second, despite high comorbidity rates reported in the literature [3, 5, 6], in our study, participant’s depression symptoms and obesity at baseline were mostly in the normal to very mild range, possibly reflecting a recruitment bias. Importantly, the low incidence of baseline depressive symptoms might have attenuated the chance of detection of intervention effects due to a floor effect. Future studies, therefore, may focus on people with ADHD with diagnoses of depression or obesity to assess efficacy of BLT and EI in terms of treatment [33]. Third, participants showed already high physical activity before start of intervention (i.e. mean number of steps per day above the recommended criterion of 10,000) [34] and most participants had medium (n = 56, 28%) or high (n = 87, 44%) cardiorespiratory fitness. Thus, exercise-based interventions may not show an additional effect in young people with ADHD [33]. Fourth, a future efficacy trial should also measure the degree of physical activity and light exposure in the TAU group given that, in general, young people may engage in exercises and outdoor activities (see Table 1 for physical activity and light exposure assessed at baseline). The lack of controlling for these factors might have reduced the chance to find between-group differences in our pilot study.
In summary, the potential of m-health technologies to promote lifestyle changes in clinical populations has been widely discussed, but empirical evidence is scarce. We developed a novel m-health system to support BLT and EI in young people with ADHD to prevent co-occurring depression and obesity. The findings of this pilot RCT, implemented with high quality in relation to observer-blind assessments, online monitoring, and sophisticated statistical analyses, reveal that both BLT and EI conducted in an individual, naturalistic setting with an m-health system were not feasible in this group. Thus, we recommend revisions to the m-health approach and behavioural interventions for young people with ADHD, implementing co-design including affected individuals and other relevant stakeholders, and taking general (e.g. regarding the usability and acceptability of the m-health system, time commitment) as well as age-related and ADHD-specific motivational issues (e.g. by implementing more structured or group settings) even more seriously into account. This would provide an important step forward on the path toward the prevention of common co-occurring conditions in young people with ADHD.
Conflict of interest
C.M.F. receives royalties for books on Autism Spectrum Disorder, Attention-Deficit/Hyperactivity Disorder and Major Depressive Disorder. She has served as advisor to Servier in relation to an epidemiological study in Autism Spectrum Disorder. A.R. receives honoraria for talks and/ or advisory boards from Janssen, SAGE/Biogen, Medice, Shire/Takeda, Boehringer Ingelheim, LivaNova, COMPASS, and cyclerion. J.A.R.Q. was on the speaker’s bureau and/ or acted as consultant for Janssen-Cilag, Shire, Takeda, Bial, Shionogi, Sincrolab, Novartis, BMS, Medice, Rubió, Uriach, Technofarma, and Raffo in the last 3 years. He also received travel awards (air tickets + hotel) for taking part in psychiatric meetings from Janssen-Cilag, Rubió, Shire, Takeda, Shionogi, Bial, and Medice. The Department of Psychiatry chaired by him received unrestricted educational and research support from the following companies in the last 3 years: Janssen-Cilag, Shire, Oryzon, Roche, Psious, and Rubió. J.K. has given talks at educational events sponsored by Medice; all funds are received by King’s College London and used for studies of ADHD. All other authors have no conflicts of interest to declare.
Ethical approval
The study protocol complied with the declaration of Helsinki (revision) and was approved by the institutional review boards of all participating centres. All participants provided written informed consent before their participation in the trial. For participants aged 14–17 years, written informed consent was also obtained from legal guardians.
Data availability
Access to a de-identified participant dataset and data dictionary is available upon reasonable request after 6 months of publication. The study protocol, statistical analysis plan, and the informed consent form can also be made available. Any such requests should be sent to the corresponding author. Requests will be assessed for scientific rigour before being granted by the trial PI. A data-sharing agreement might be required. Data will be anonymised and securely transferred.
References
Franke B, Michelini G, Asherson P, Banaschewski T, Bilbow A, Buitelaar JK, Cormand B, Faraone SV, Ginsberg Y, Haavik J, Kuntsi J, Larsson H, Lesch K-P, Ramos-Quiroga JA, Réthelyi JM, Ribases M, Reif A (2018) Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur Neuropsychopharmacol 28(10):1059–1088. https://doi.org/10.1016/j.euroneuro.2018.08.001
Kittel-Schneider S, Arteaga-Henriquez G, Vasquez AA, Asherson P, Banaschewski T, Brikell I, Buitelaar J, Cormand B, Faraone SV, Freitag CM, Ginsberg Y, Haavik J, Hartman CA, Kuntsi J, Larsson H, Matura S, McNeill RV, Ramos-Quiroga JA, Ribases M, Romanos M, Vainieri I, Franke B, Reif A (2022) Non-mental diseases associated with ADHD across the lifespan: Fidgety Philipp and Pippi Longstocking at risk of multimorbidity? Neurosci Biobehav Rev 132:1157–1180. https://doi.org/10.1016/j.neubiorev.2021.10.035
Libutzki B, Ludwig S, May M, Jacobsen RH, Reif A, Hartman CA (2019) Direct medical costs of ADHD and its comorbid conditions on basis of a claims data analysis. Eur Psychiatry 58:38–44. https://doi.org/10.1016/j.eurpsy.2019.01.019
Biederman J, Ball SW, Monuteaux MC, Mick E, Spencer TJ, McCREARY M, Cote M, Faraone SV (2008) New insights into the comorbidity between ADHD and major depression in adolescent and young adult females. J Am Acad Child Adolesc Psychiatry 47(4):426–434. https://doi.org/10.1097/CHI.0b013e31816429d3
Jacob CP, Romanos J, Dempfle A, Heine M, Windemuth-Kieselbach C, Kruse A, Reif A, Walitza S, Romanos M, Strobel A, Brocke B, Schäfer H, Schmidtke A, Böning J, Lesch K-P (2007) Co-morbidity of adult attention-deficit/hyperactivity disorder with focus on personality traits and related disorders in a tertiary referral center. Eur Arch Psychiatry Clin Neurosci 257(6):309–317. https://doi.org/10.1007/s00406-007-0722-6
Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, Faraone SV (2016) Association between ADHD and obesity: a systematic review and meta-analysis. Am J Psychiatry 173(1):34–43. https://doi.org/10.1176/appi.ajp.2015.15020266
Meinzer MC, Lewinsohn PM, Pettit JW, Seeley JR, Gau JM, Chronis-Tuscano A, Waxmonsky JG (2013) Attention-deficit/hyperactivity disorder in adolescence predicts onset of major depressive disorder through early adulthood. Depress Anxiety 30(6):546–553. https://doi.org/10.1002/da.22082
Philipsen A, Jans T, Graf E, Matthies S, Borel P, Colla M, Gentschow L, Langner D, Jacob C, Groß-Lesch S, Sobanski E, Alm B, Schumacher-Stien M, Roesler M, Retz W, Retz-Junginger P, Kis B, Abdel-Hamid M, Heinrich V, Huss M, Kornmann C, Bürger A, Perlov E, Ihorst G, Schlander M, Berger M, van Tebartz EL (2015) Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiat 72(12):1199–1210. https://doi.org/10.1001/jamapsychiatry.2015.2146
Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR (2009) The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry 48(5):484–500. https://doi.org/10.1097/CHI.0b013e31819c23d0
Ortega FB, Cadenas-Sanchez C, Migueles JH, Labayen I, Ruiz JR, Sui X, Blair SN, Martínez-Vizcaino V, Lavie CJ (2018) Role of physical activity and fitness in the characterization and prognosis of the metabolically healthy obesity phenotype: a systematic review and meta-analysis. Prog Cardiovasc Dis 61(2):190–205. https://doi.org/10.1016/j.pcad.2018.07.008
Brown T, Moore TH, Hooper L, Gao Y, Zayegh A, Ijaz S, Elwenspoek M, Foxen SC, Magee L, O’Malley C, Waters E, Summerbell CD (2019) Interventions for preventing obesity in children. Cochrane Database Syst Rev 7:001871. https://doi.org/10.1002/14651858.CD001871.pub4
Bailey AP, Hetrick SE, Rosenbaum S, Purcell R, Parker AG (2018) Treating depression with physical activity in adolescents and young adults: a systematic review and meta-analysis of randomised controlled trials. Psychol Med 48(7):1068–1083. https://doi.org/10.1017/S0033291717002653
Pearce M, Garcia L, Abbas A, Strain T, Schuch FB, Golubic R, Kelly P, Khan S, Utukuri M, Laird Y, Mok A, Smith A, Tainio M, Brage S, Woodcock J (2022) Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiat. https://doi.org/10.1001/jamapsychiatry.2022.0609
Schuch FB, Stubbs B (2019) The role of exercise in preventing and treating depression. Curr Sports Med Rep 18(8):299–304. https://doi.org/10.1249/JSR.0000000000000620
Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, Hallgren M, Ponce De Leon A, Dunn AL, Deslandes AC, Fleck MP, Carvalho AF, Stubbs B (2018) Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry 175(7):631–648. https://doi.org/10.1176/appi.ajp.2018.17111194
Grimm O, van Rooij D, Hoogman M, Klein M, Buitelaar J, Franke B, Reif A, Plichta MM (2021) Transdiagnostic neuroimaging of reward system phenotypes in ADHD and comorbid disorders. Neurosci Biobehav Rev 128:165–181. https://doi.org/10.1016/j.neubiorev.2021.06.025
Korman M, Palm D, Uzoni A, Faltraco F, Tucha O, Thome J, Coogan AN (2020) ADHD 24/7: Circadian clock genes, chronotherapy and sleep/wake cycle insufficiencies in ADHD. World J Biol Psychiatry 21(3):156–171. https://doi.org/10.1080/15622975.2018.1523565
Vogel SWN, Bijlenga D, Tanke M, Bron TI, van der Heijden KB, Swaab H, Beekman ATF, Kooij JJS (2015) Circadian rhythm disruption as a link between attention-deficit/hyperactivity disorder and obesity? J Psychosom Res 79(5):443–450. https://doi.org/10.1016/j.jpsychores.2015.10.002
Al-Karawi D, Jubair L (2016) Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J Affect Disord 198:64–71. https://doi.org/10.1016/j.jad.2016.03.016
Beauchamp MT, Lundgren JD (2016) A systematic review of bright light therapy for eating disorders. Prim Care Companion CNS disorders. https://doi.org/10.4088/PCC.16r02008
Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, Dumuid D, Kasai D, O’Connor E, Virgara R, Maher C (2022) Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digital Health 4(8):e615–e626. https://doi.org/10.1016/S2589-7500(22)00111-X
Lee AM, Chavez S, Bian J, Thompson LA, Gurka MJ, Williamson VG, Modave F (2019) Efficacy and effectiveness of mobile health technologies for facilitating physical activity in adolescents: scoping review. JMIR Mhealth Uhealth 7(2):e11847. https://doi.org/10.2196/11847
Schoenfelder E, Moreno M, Wilner M, Whitlock KB, Mendoza JA (2017) Piloting a mobile health intervention to increase physical activity for adolescents with ADHD. Prev Med Rep 6:210–213. https://doi.org/10.1016/j.pmedr.2017.03.003
Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE (2013) Exercise for depression. Cochrane Database Syst Rev 9:CD004366. https://doi.org/10.1002/14651858.CD004366.pub6
Pjrek E, Friedrich M-E, Cambioli L, Dold M, Jäger F, Komorowski A, Lanzenberger R, Kasper S, Winkler D (2020) The efficacy of light therapy in the treatment of seasonal affective disorder: a meta-analysis of randomized controlled trials. Psychother Psychosom 89(1):17–24. https://doi.org/10.1159/000502891
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA (2016) CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 355:i5239. https://doi.org/10.1136/bmj.i5239
Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, Bond CM (2016) Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS ONE 11(3):e0150205. https://doi.org/10.1371/journal.pone.0150205
McGrattan AM, McEvoy CT, Vijayakumar A, Moore SE, Neville CE, McGuinness B, McKinley MC, Woodside JV (2021) A mixed methods pilot randomised controlled trial to develop and evaluate the feasibility of a Mediterranean diet and lifestyle education intervention “THINK-MED” among people with cognitive impairment. Pilot Feasibility Stud 7(1):3. https://doi.org/10.1186/s40814-020-00738-3
Morrison AP, Pyle M, Maughan D, Johns L, Freeman D, Broome MR, Husain N, Fowler D, Hudson J, MacLennan G, Norrie J, Shiers D, Hollis C, James A (2020) Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry 7(9):788–800. https://doi.org/10.1016/S2215-0366(20)30248-0
Mayer JS, Hees K, Medda J, Grimm O, Asherson P, Bellina M, Colla M, Ibáñez P, Koch E, Martinez-Nicolas A, Muntaner-Mas A, Rommel A, Rommelse N, de Ruiter S, Ebner-Priemer UW, Kieser M, Ortega FB, Thome J, Buitelaar JK, Kuntsi J, Ramos-Quiroga JA, Reif A, Freitag CM (2018) Bright light therapy versus physical exercise to prevent co-morbid depression and obesity in adolescents and young adults with attention-deficit / hyperactivity disorder: study protocol for a randomized controlled trial. Trials 19(1):140. https://doi.org/10.1186/s13063-017-2426-1
Halvorsrud K, Kucharska J, Adlington K, Rüdell K, Brown Hajdukova E, Nazroo J, Haarmans M, Rhodes J, Bhui K (2019) Identifying evidence of effectiveness in the co-creation of research: a systematic review and meta-analysis of the international healthcare literature. J Public Health 43(1):197–208. https://doi.org/10.1093/pubmed/fdz126
Jones RB, Stallard P, Agha SS, Rice S, Werner-Seidler A, Stasiak K, Kahn J, Simpson SA, Alvarez-Jimenez M, Rice F, Evans R, Merry S (2020) Practitioner review: Co-design of digital mental health technologies with children and young people. J Child Psychol Psychiatry 61(8):928–940. https://doi.org/10.1111/jcpp.13258
Sun W, Yu M, Zhou X (2022) Effects of physical exercise on attention deficit and other major symptoms in children with ADHD: A meta-analysis. Psychiatry Res 311:114509. https://doi.org/10.1016/j.psychres.2022.114509
Paluch AE, Bajpai S, Bassett DR, Carnethon MR, Ekelund U, Evenson KR, Galuska DA, Jefferis BJ, Kraus WE, Lee I-M, Matthews CE, Omura JD, Patel AV, Pieper CF, Rees-Punia E, Dallmeier D, Klenk J, Whincup PH, Dooley EE, Gabriel KP, Palta P, Pompeii LA, Chernofsky A, Larson MG, Vasan RS, Spartano N, Ballin M, Nordström P, Nordström A, Anderssen SA, Hansen BH, Cochrane JA, Dwyer T, Wang J, Ferrucci L, Liu F, Schrack J, Urbanek J, Saint-Maurice PF, Yamamoto N, Yoshitake Y, Newton RL, Yang S, Shiroma EJ, Fulton JE (2022) Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public health 7(3):e219–e228. https://doi.org/10.1016/S2468-2667(21)00302-9
Acknowledgements
We are very grateful to Anna Rommel for setting up the study at King’s College London, and to the Data Safety and Monitoring Board (Wolfgang Retz, Michael Rösler, Peter Schlattmann) and the Coordination Centre for Clinical Trials Heidelberg (Karsten Thelen and Anna-Lena Gamer) for their support and advice. We thank the clinicians of the (child and adolescent) psychiatric and medical departments of the University Hospital Frankfurt (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy and Department of Psychiatry, Psychosomatic Medicine and Psychotherapy), the Hospital Universitari Vall d’Hebron Barcelona (Department of Mental Health), the Springfield University Hospital London, and the Radboud University Nijmegen (Department of Psychiatry and Karakter Child and Adolescent Psychiatry) for the great collaboration with the study teams at Frankfurt, Barcelona, London, and Nijmegen. Marietta Kirchner, Institute of Medical Biometry, University of Heidelberg, Germany, has provided additional data analyses regarding possible factors helping to explain the low intervention adherence of our study population.
Funding
Open Access funding enabled and organized by Projekt DEAL. The research has received funding from the EU Framework Programme for Research and Innovation, Horizon 2020 under grant agreement no. 667303 (CoCA). F.B.O. research activity was also supported by the University of Granada, Plan Propio de Investigación 2016, Excellence actions: Unit of Excellence on Exercise, Nutrition and Health (UCEENS). The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
C.M.F. is the study PI. C.M.F. developed the study design and A.R., J.A.R.Q., J.K.B., J.K., P.A., O.G., J.S., M.K., E.K., U.E.P., A.M.-M., F.B.O., and M.C. contributed to discussions relevant to the study design. C.M.F. and J.S.M. finalised and published the study protocol with input from A.R., J.A.R.Q., J.K.B., J.K., P.A., O.G., J.S., M.K., E.K., U.E.P., A.M.-M., F.B.O., and M.C.. The intervention manual on bright light therapy was developed by M.C., who also supervised its implementation throughout the trial. A.M.-M. and F.B.O. developed the exercise intervention manual and supervised its implementation throughout the trial. The mobile health app was developed by E.K. and U.E.P. who also supervised its use throughout the trial. The local PIs of the study are C.M.F. and A.R. (Frankfurt), J.A.R.Q. (Barcelona), J.K.B. (Nijmegen), and J.K. (London). Recruitment and data acquisition was performed by J.S.M., J.M., G.B., O.G. (Frankfurt), J.P.S., V.R. (Barcelona), D.B. (Nijmegen), and A.D.P. (London). J.S. and L.K. were responsible for data management comprising all tasks concerning acquisition, processing, and utilisation of the trial data. W.R. supervised study progress throughout the trial. J.S.M., C.M.F., L.K., M.K. developed the statistical analysis plan. L.K. and M.K. did the statistical analysis. J.S.M., C.M.F., L.K., J.S., and M.K. accessed and verified the data. Pre-processing of m-health data was done by E.K. and U.E.P. C.M.F. and J.S.M. interpreted the analyses with input from all the authors and wrote a first draft of the paper. All authors reviewed and approved the final manuscript.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mayer, J.S., Kohlhas, L., Stermann, J. et al. Bright light therapy versus physical exercise to prevent co-occurring depression in adolescents and young adults with attention-deficit/hyperactivity disorder: a multicentre, three-arm, randomised controlled, pilot phase-IIa trial. Eur Arch Psychiatry Clin Neurosci (2024). https://doi.org/10.1007/s00406-024-01784-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-024-01784-1