Abstract
Background
Understanding the relationship between psychopathology and major domains of human neurobehavioral functioning may identify new transdiagnostic treatment targets. However, studies examining the interrelationship between psychopathological symptoms, sensorimotor, cognitive, and global functioning in a transdiagnostic sample are lacking. We hypothesized a close relationship between sensorimotor and cognitive functioning in a transdiagnostic patient sample.
Methods
We applied network analysis and community detection methods to examine the interplay and centrality [expected influence (EI) and strength] between psychopathological symptoms, sensorimotor, cognitive, and global functioning in a transdiagnostic sample consisting of 174 schizophrenia spectrum (SSD) and 38 mood disorder (MOD) patients. All patients (n = 212) were examined with the Positive and Negative Syndrome Scale (PANSS), the Heidelberg Neurological Soft Signs Scale (NSS), the Global Assessment of Functioning (GAF), and the Brief Cognitive Assessment Tool for Schizophrenia consisted of trail making test B (TMT-B), category fluency (CF) and digit symbol substitution test (DSST).
Results
NSS showed closer connections with TMT-B, CF, and DSST than with GAF and PANSS. DSST, PANSS general, and NSS motor coordination scores showed the highest EI. Sensory integration, DSST, and CF showed the highest strength.
Conclusions
The close connection between sensorimotor and cognitive impairment as well as the high centrality of sensorimotor symptoms suggests that both domains share aspects of SSD and MOD pathophysiology. But, because the majority of the study population was diagnosed with SSD, the question as to whether sensorimotor symptoms are really a transdiagnostic therapeutic target needs to be examined in future studies including more balanced diagnostic groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The co-occurrence of psychopathological symptoms stemming from different functional domains is characteristic of psychiatric disorders. For instance, schizophrenia spectrum disorders (SSD) are characterized by psychopathological symptoms from various functional domains, including sensory–perceptual (e.g., hallucinations), cognitive (e.g., delusions, impaired working memory and executive functioning), affective (e.g., flat affect, anhedonia and depressive symptomatology [1]), somatic (e.g., fatigue, muscle pain, gastrointestinal symptoms and tension [2]), social (e.g., social isolation and autism), and sensorimotor (e.g., rigor, tremor, psychomotor slowing and akinesia [3]), respectively. In mood disorders (MOD), such as major depressive disorder (MDD) and bipolar disorder (BD), patients not only experience low mood, sadness [4], low self-esteem, lack of drive, and loss of interest or pleasure but also co-occurring cognitive changes (e.g., rumination [5] and impaired working memory [6]), somatic-neurovegetative (chest pain, gastrointestinal symptoms, irritable bowel, or other [7]), interpersonal (e.g., social anxiety and withdrawal [8]), sensory–perceptual (aberrant visual perception [9]), and motor (psychomotor retardation or agitation [10]) symptoms.
Furthermore, the majority of the psychopathological symptoms mentioned above are core diagnostic criteria for SSD and MOD by the most widely used diagnostic systems in clinical practice, such as the DSM-5 and ICD-11. The overlap and co-occurrence of psychopathological symptoms in SSD and MOD are specifically mentioned in both diagnostic systems as the foundation for differential diagnostic considerations. However, their intrinsic relationships—that is, in what ways do the various psychopathological symptoms interact and which symptoms are central or influential across SSD and MOD—remain understudied. In the last few years, novel statistical methods have been developed to explore the interplay between psychopathological symptoms and global functioning in psychiatric disorders [11,12,13]. In particular, network analysis can help identifying which psychopathological symptoms are central or influential within a network of different clinical variables [14, 15]. Central psychopathological symptoms are those that have strong connections to other symptoms or clinical variables and may play a crucial role in the development and maintenance of the disorder. Therefore, network analysis is a promising method to investigate the complexity of interactions between psychopathological symptoms stemming from different functional domains across different psychiatric disorders. Instead of seeing psychopathological symptoms as standalone entities, network analysis conceives of them as components of a complex network that interact with one another and frequently reinforce one another. In line with this, psychiatric disorders, such as SSD and MOD, might arise from the direct interactions of symptoms within a network architecture.
In the last two years, the network analysis method has contributed to a considerable increase in knowledge in the field of different psychopathological symptoms [16,17,18]. However, the studies using network analysis method have investigated mainly the interaction between different categories of psychopathological symptoms using single items stemming from different rating scales such as the Brief Negative Symptom Scale (BNSS) or the Positive and Negative Syndrome Scale (PANSS) in uni-diagnostic psychiatric samples [16,17,18].
Based on the recent evidence that distinct Neurological Soft Signs (NSS) have been shown to be differentially associated with psychopathology as well as cognition in SSD [19,20,21] and BD [22], the main goal of this study was to examine the associations among psychopathological symptoms, sensorimotor, cognitive, and global functioning in a transdiagnostic patient sample. We hypothesized that sensorimotor dysfunction, as examined with the Heidelberg NSS scale [23] would be more closely connected with cognition (as assessed with the Brief Cognitive Assessment Tool for Schizophrenia [B-CATS] [24]) than psychopathological symptoms examined with PANSS. Further, we hypothesized that sensorimotor dysfunction would be a central network component (as assessed with expected influence [EI] and strength). Unlike previous studies that conducted the network analyses at the single-item level, and because we had a strong prior theory on how variables are related, we decided to use subscale scores in order to examine associations between subdomains. If we confirmed our hypotheses, this study could point toward shared domain-based aspects of pathophysiology in SSD and MOD. This could point toward new treatment options for cognition, e.g., with stimulation techniques in specific brain regions associated with sensorimotor dysfunction [25, 26] or virtual reality [27].
Methods
Study participants
In this study, we combined two independent cohorts of patients from different studies conducted at the Central Institute of Mental Health (CIMH).
Cohort #1 consisted of 129 subjects fulfilling the DSM-IV-TR [28] criteria for schizophrenia spectrum disorders (SSD) and 7 subjects fulfilling the DSM-IV-TR [28] criteria for bipolar disorders (BD) [29, 30]. This cohort has been used in previous studies of our group [3, 31, 32]. Diagnoses were made by staff psychiatrists and confirmed using the German versions of the Structured Clinical Interview for DSM-IV-TR axis I and II disorders (SCID) and examination of the case notes (SF and DH).
Cohort #2 consisted of 76 subjects fulfilling the German Mini Diagnostic Interview for Mental Disorders (Mini-DIPS) [33] criteria for SSD (n = 45), major depressive disorder (MDD, n = 26) or BD (n = 5). Diagnoses were made by staff psychiatrists and confirmed using the examination of the case notes (SF, GAB and DH).
Patients in both cohorts were excluded if: (i) they were aged < 18 or > 65 years; (ii) they had a history of brain trauma or neurological disease (especially movement disorders); (iii) they had shown alcohol/substance use disorder within 12 months prior to participation.
The local Ethics Committees I and II (Medical Faculty Heidelberg and Medical Faculty Mannheim at Heidelberg University, Germany) approved the studies. We obtained written informed consent from all study participants after all aims and procedures of the study had been fully explained.
Clinical assessment
Patients in both cohorts were examined during in- or outpatient treatment after partial remission of acute psychopathological symptoms. All relevant study procedures (e.g., psychopathological rating scales, neuropsychological assessments and sensorimotor assessment) were completed within 7 days. All SSD and MOD patients were on a stable daily dose of antipsychotic, antidepressant or mood-stabilizing medication for at least 7 days. Antipsychotic and antidepressant medication was standardized as Olanzapine [OLZe] [34] and fluoxetine [FLX] [35] equivalents (s. Table 1 for details). OLZe and FLXe were z-transformed, summed up and included as covariates (medication) in subsequent analyses (see below).
Patients were examined with the PANSS [36] for psychopathological assessment, including the following PANSS subscores: positive, negative and general. The Heidelberg NSS scale [23] including its sub-scores motor coordination (MOCO), sensory integration (SI), hard signs (HS), complex motor tasks (COMT), right/left spatial orientation (RLSPO) was used for sensorimotor assessment. General functioning was assessed with the Global Assessment of Functioning (GAF) scale [37]. Cognitive functioning was assessed with B-CATS [24], which consisted of trail making test B (TMT-B), category fluency (CF), and digit symbol substitution test (DSST). One of the main prerequisites for this study was to obtain the largest possible sample with overlapping psychopathological, sensorimotor, cognitive, and global assessments. Therefore, we opted for scales/assessments that are feasible and map the four domains we were interested in. Further, we decided on scales/assessments that have been previously used in the three disorders [38,39,40]. Therefore, PANSS, NSS, B-CATS, and GAF seemed to us to be the most suitable. Finally, we believe that the included scales PANSS, NSS, B-CATS, and GAF also allow good comparability with other studies.
Statistical analyses
We used R version 4.0.4 and Rstudio version 1.3.1093 [41]. Initially, a descriptive analysis of demographic and clinical data was performed. Then, the homogeneity of variances and the normality of PANSS, NSS, GAF, and B-CATS were investigated using Levene’s test and Shapiro–Wilk. Further analyses were performed with PANSS sub-scores (positive, negative, and general), NSS sub-scores (MOCO, HS, COMT, RLSPO, SI), GAF score, and B-CATS sub-scores (TMT-B, DSST, CF). We used sub-scores in order to examine differential associations between subdomains rather than broad global scores which may mask associations. Since higher values of the variables GAF, CF, and DSST indicated better performance (while on the remaining scales, higher values indicated worse performance), GAF, CF, and DSST were converted reversely.
Using these scores, we have carried out the following statistical analyses: First, in line with previously published studies [14, 42,43,44], potentially relevant covariates (regression variables), such as age, sex, education, and medication, were not included in our analyses. Second, for completeness, we (A) regressed out age, sex, education, and medication and employed the residuals of PANSS sub-scores, NSS subscale scores, B-CATS sub-scores, and GAF score for network estimation and (B) included age, sex, education, and medication nodes in the network analyses (NA2-A and NA2-B) and EGA.
In the first and second step, we employed network analysis methods [45, 46]. In this approach, nodes represent variables, and lines between variables are termed edges, representing relationships to be investigated. The most common psychological network estimation technique uses partial correlations between variables (nodes), which are calculated after accounting for all other variable correlations. Blue edges refer to positive correlations, while red edges refer to negative correlations. Thicker edges refer to stronger correlations, while thinner edges refer to weaker correlations. Due to the violation of normality in our variables, we used a non-paranormal transformation in preparing the data. Employing the qgraph package, we set the default option of the estimateNetwork function to “huge”. Network estimation involved a graphical Gaussian model (GGM) [45, 47]. We applied the extended Bayesian information criterion (EBIC) [47, 48]. To avoid false-positive relationships between nodes, we regularized the network with the least absolute shrinkage and selection operator (LASSO) [47, 48]. LASSO can shrink small edges toward zero. Fruchterman–Reingold algorithm was used for visualization [48]. This algorithm places nodes with stronger correlations more centrally. To assess centrality, expected influence (EI) and strength were calculated. EI of a node represents the sum of all partial correlations (accounting for negative edges) and indicates its importance within a network graph. Higher values indicate stronger interconnectedness. Strength is the sum of absolute edge weights. To examine EI and strength stability, we employed a bootstrap approach with the bootnet package. Furthermore, and in order to investigate whether our results may have been driven by diagnosis, we repeated the network analyses including only SSD participants (n = 174, s. Supplementary Figs. 6–12). We refrained from repeating the analyses including only MOD patients (BD: n = 12; MDD: n = 24) or analyzing the two study cohorts (NSS cohort: n = 129; whiteCAT cohort: n = 83) separately, due to the small number of participants in terms of network analysis.
Then, in order to employ community detection methods on PANSS, NSS, GAF, and B-CATS, we used exploratory graph analysis (EGA) [49,50,51]. To this end, we employed the EGAnet package. EGA calculates polychoric correlations with the walktrap algorithm in order to identify communities of the partial correlation matrix [49]. The walktrap algorithm calculates similarities between vertices hinged on random walks [49]. Additionally, item stability was evaluated with 1000 bootstrapped samples, assessing the proportion of times specific items clustered with their community. Network loadings for each community were investigated with EGAnet. Loadings are interpreted as the node´s expected influence on each community. Higher loadings indicate that a node has a higher influence on the occurrence of a community.
Third, to validate the results of the network analyses, a partial correlation (C) was run to determine the relationship between PANSS positive, negative and general score, NSS subscale scores, B-CATS subscores, and GAF score while controlling for age, sex, education, and medication.
Results
Clinical and demographic characteristics
The study cohort (n = 212) consisted of participants with SSD (n = 174), MDD (n = 26) and BD (n = 12). Mean age and education were 37.88 years and 13.28 years, respectively. There were 104 male and 108 female participants. Detailed demographic and clinical characteristics of participants across all diagnoses and the included variables as well as skewness and kurtosis for each variable are shown in Table 1 (for group differences between variables see Supplementary Table 1).
Network analysis
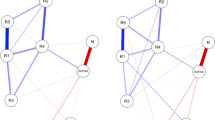
First, the network included the NSS, the PANSS, and the B-CATS sub-scores and GAF total scores (Fig. 1). Edges as well as expected influence (EI) and strength were deemed stable (s. Supplementary Figs. 1 and 2). The scores of the five NSS subscales showed low to no association with PANSS positive, negative, and general scores (Fig. 1, Table 2). MOCO showed associations with the three B-CATS sub-scores (Fig. 1, Table 2). SI showed an inverse association with CF and a positive association with TMT-B (Fig. 1, Table 2). The remaining NSS sub-scores showed fewer associations with B-CATS sub-scores (Fig. 1, Table 2). GAF was associated with PANSS scores and NSS IF sub-scores (Fig. 1, Table 2). DSST, PANSS general, MOCO, and TMT-B showed the largest EI as a measure of centrality (Fig. 2). EI of MOCO was not significantly different from DSST, PANSS general, or TMT-B EI (s. Supplementary Fig. 3), indicating similar centrality. Also, SI, DSST, and CF showed the highest strength as another measure of centrality, followed by PANSS general and MOCO (Fig. 2). SI strength was not significantly different from strength of either DSST, CF, PANSS general, MOCO, or TMT-B, indicating similar centrality (s. Supplementary Fig. 3).
This figure describes the methodological approach of the study including the network structures of psychopathological symptoms, sensorimotor, cognitive, and global functioning in a transdiagnostic sample. Node colors in the estimated multi-dimensional network reflect the four functional domains. Blue edges represent positive associations; red edges represent negative associations. Thickness and saturation of edges indicate the strength of these associations
Centrality of the estimated multi-domain network. DSST: digit symbol substitution test; G: PANSS, general psychopathology dimension; MOCO: Neurological Soft Signs—Motor Coordination; TMT: Trail Making Test B; N: PANSS, negative symptoms; COMT: Neurological Soft Signs—Complex motor tasks; GAF: Global Assessment of Functioning; SI: Neurological Soft Signs—Sensory Integration subscale; P: PANSS, positive symptoms; RLSPO: Neurological Soft Signs—Right/Left and Spatial Orientation; CF: Category Fluency; HS: Neurological Soft Signs – Hard Signs
Second, the network (NA2-A) estimated after regressing out age, sex, education, and medication was unstable and thus could not be interpreted and the results are not shown. Unstable in this context refers to low probability of being able to reproduce the estimated network structure, including edges. Further, the network analysis (NA2-B) including age, sex, education, and medication as additional nodes was sufficiently stable (s. Supplementary Figs. 15 and 16) and the results remained comparable to our network analysis without these four variables (NA1): NSS still showed closer connections with TMT-B, CF and DSST than with GAF and PANSS (s. Supplementary Fig. 13 and supplementary Table 2). PANSS general, DSST, TMT, and NSS MOCO scores showed the highest EI, while DSST, SI, and TMT showed the highest strength (s. Supplementary Fig. 14). Overall, age, sex, education, and medication showed low centrality (s. Supplementary Fig. 14).
Community detection
Bootstrapped EGA revealed three communities (Fig. 3): cluster #1 included GAF total score and the three PANSS subscores, cluster #2 included five NSS subscores, cluster #3 included TMT-B, DSST and CF. Item replicability was sufficient in cluster #1 and #2, yet there was low replicability in cluster #3 (Table 3). Bootstrapping dimension frequency was 0.57 for two factors and 0.39 for three factors, the median dimension was 2, and the confidence interval was 0.85–3.15. Network loadings are shown in Table 4. In total, the EGA results were deemed unstable and therefore, we refrained from repeating the EGA including age, sex, education, and medication.
This figure highlights the bootstrapped exploratory graph analysis. This analysis revealed three communities: Cluster #1 (red) included GAF total score and the three PANSS subscores. Cluster #2 (blue) included five NSS subscores. Cluster #3 (light green) included TMT-B, DSST and CF. DSST: digit symbol substitution test; G: PANSS, general psychopathology dimension; MOCO: Neurological Soft Signs—Motor Coordination; TMT: Trail Making Test B; N: PANSS, negative psychosis dimension; COMT: Neurological Soft Signs—Complex motor tasks; GAF: Global Assessment of Functioning; SI: Neurological Soft Signs—Sensory Integration subscale; P: PANSS, positive psychosis dimension; RLSPO: Neurological Soft Signs—Right/Left and Spatial Orientation; CF: Category Fluency; HS: Neurological Soft Signs—Hard Signs
Covariate-adjusted correlation analysis
The partial correlation (C) (after log-transformation of variables that were not normally distributed) revealed that CF was significantly associated with NSS MOCO as well as NSS SI while controlling for all other variables (r = 0.25, p < 0.001 and r = − 0.33, p < 0.001, respectively; uncorrected for multiple testing). Also, TMT-B was associated with NSS SI while controlling for all other variables (r = 0.28, p < 0.001; uncorrected for multiple testing). Notably, PANSS scores were not significantly associated with NSS scores (uncorrected for multiple testing).
Discussion
Using the network approach to psychopathology, we investigated—for the first time—the interrelationship between psychopathological symptoms, sensorimotor, cognitive, and global functioning in a transdiagnostic sample consisting of SSD, MDD, and BD patients. Two main findings emerged: First, NSS showed closer connections with TMT-B, CF and DSST than with GAF and PANSS. Second, DSST, PANSS general, and NSS MOCO scores showed the highest EI, while SI, DSST, and CF showed the highest strength. However, the EGA was unstable and could not be interpreted.
The first finding supports and extends our understanding of the relationship between sensorimotor and cognitive domains for several reasons: First, there has been prior research linking more severe hypokinetic sensorimotor abnormalities (e.g., NSS and parkinsonism) to executive functioning deficiencies in SSD [52, 53]. Second, our findings are in line with Wolf et al. [54] and confirm the precise link between the sensorimotor and cognitive domains. Our findings extend this previous work transdiagnostically. Third, the TMT-B and DSST assessments are well-known tools of cognitive performance, particularly in terms of processing speed, cognitive flexibility, and the phenomenon known as psychomotor slowing. This line of reasoning suggests that a more inclusive definition of this term may incorporate sensorimotor disorders such as NSS [55]. Accordingly, Osborne et al. [55] described that the cognitive (“psycho”) and sensorimotor sub-processes that lead to psychomotor slowing may overlap with the sub-processes of subtle sensorimotor abnormalities such as NSS. Finally, NSS and TMT-B are associated with overlapping circuitry, suggesting a biological link between sensorimotor and cognitive processes [56]. On one side, changes in the inferior frontal gyrus, paracentral gyrus, inferior parietal lobe (IPL), bilateral putamen, cerebellum, and both the superior and middle temporal gyri (STG and MTG) are associated with NSS (for a summary, see also Hirjak et al. [57, 58] and Zhao et al. [59]). On the other side, the prefrontal cortex, the IPL, and the cerebellum play also a crucial role in the execution of TMT-B [60]. This implies that a shared neurobiological process may lead to manifest sensorimotor and cognitive symptoms in SSD and MOD. Our results together with the above mentioned evidence extend our current understanding of interrelated behavior in the RDoC Matrix.
Last but not least, although there have been reports—also from our group [19]—examining the bilateral link between NSS and psychopathology as well as NSS and cognition [20, 21], the precise trilateral relationship between cognition, NSS, and psychopathology remains unclear. We interpret our results as suggesting that sensorimotor symptoms are closer to cognition than psychopathology, but this relationship does not exclude connections between NSS and psychopathology. Indeed, as shown in Table 2, PANSS positive and negative subscales showed (rather weak) partial correlations with NSS subscales. Psychopathology also showed few connections with cognitive symptoms, which is in line with assumptions that the psychopathology domain by itself (as outcome parameter) only incompletely reflects functional impairment, which is more closely connected with cognitive deficits [61].
Second, the most central nodes in terms of EI were DSST, PANSS general, and NSS MOCO scores. All nodes showed a positive value in the centrality index. In terms of strength, SI, DSST and CF showed the highest centrality. These findings are crucial for a number of reasons: First, the DSST examines processing speed, working memory, visuospatial processing, and attention. Also, another recent network analysis in SSD showed a central place for processing speed evaluated with symbol coding [42]. This said, DSST reflects the global cognitive functioning, which, when impaired, can lead to disturbances at the level of sensorimotor functions. In line with this, Morrens et al. [62] used DSST (e.g., matching time and writing time) to examine sensorimotor and cognitive slowing in SSD. Although the authors concluded that both processes are unrelated, matching time was associated with neuropsychological test results. Second, this fits in well, because MOCO also plays a central role in our network. The MOCO subscale includes five items, such as Ozeretski’s test, diadochokinesia, pronation/supination, finger-to-thumb opposition, and speech articulation. Both DSST and MOCO are based on movement execution, predominantly at the level of sensorimotor and visuospatial control. Third, disturbances of sensorimotor and spatial–visual control can lead to various psychopathological symptoms, such as somatic concerns, anxiety, depression, motor retardation, disorientation, disturbance of volition, poor impulsive control, and preoccupation, respectively [63, 64]. This could be a possible explanation for the centrality of the PANSS general scores. Fourth, as proposed by the European consensus on assessments and perspectives [26], new treatment targets for stimulation techniques need to be identified. From a network point of view, variables are interrelated and targeting the most central nodes could affect other nodes as well. Consequently the connection between sensorimotor symptoms and cognition and the high centrality of sensorimotor symptoms suggest that sensorimotor symptoms and cognitive symptoms may share aspects of pathophysiology. Specifically, the high centrality of MOCO and SI together with the previously identified neurobiological correlates of NSS may suggest NSS MOCO and SI as possible treatment targets for stimulation techniques (such as transcranial direct current stimulation or repetitive transcranial magnetic stimulation) in order to improve cognition. In addition, one may wonder whether movement exercises that train the individual items of the two subscales could also lead to an improvement in cognition. This is in accordance with previous reports showing associations between NSS and cognition in longitudinal investigations [20]. Previously reported structural correlates of NSS [25] would be the primary neurobiological target regions for these stimulation techniques. This train of thought seems particularly relevant since cognitive deficits in SSD are associated with lower global and social functioning [61, 65] and are difficult to treat so far. Yet, while structural correlates of NSS have been repeatedly demonstrated in SSD [25, 66,67,68,69], we are not aware of such data in BD or MDD. When interpreting our results, it is important to bear in mind that there may not be a direct link between our non-direct, non-causal network analysis and neurobiological pathophysiology.
Taken together, complex phenomena (such as cognitive, psychopathological, and sensorimotor symptoms) may be best described at the systems level [13, 70,71,72]. More precisely, a shift from focusing on individual components to studying the organization of the system’s components seems promising [13, 70,71,72]. Network analysis provides tools to identify a system’s components and their relationships [70]. In this context, the particular benefit of network analysis techniques may be provided by their focus on patterns of pairwise conditional relationships as well as by enabling powerful visualizations of those patterns [70]. Furthermore, previous studies have established relationships between the sensorimotor and cognitive domain [52, 53]. However, the methods applied in these studies were limited: they were not able to simultaneously take into account the dynamic effects of other relevant variables on the relationship of interest as network analysis can. Also, associations between the sensorimotor and psychopathological domains have been reported as well [19]. Yet, how these domains interact simultaneously with each other has not been investigated so far. Here, our network analysis for the first time models the interactions between the sensorimotor and cognitive domains while accounting for psychopathological symptoms and global functioning. Our results suggest that there is a closer connection between the sensorimotor and cognitive domains than between the sensorimotor and psychopathological domains. Furthermore, the reported association between the sensorimotor and cognitive domains after accounting for psychopathological symptoms and global functioning may implicate several future investigative steps: First, the close connection between the sensorimotor and cognitive domains on the level of clinical tests suggest overlapping pathophysiology between both constructs [25, 60, 73]. Longitudinal MRI studies with several network analyses sequentially excluding psychopathological symptoms and global functioning may shed more light on the precise relationship between sensorimotor and cognitive symptoms. Another road may be—in accordance with previous network analysis literature [70, 71, 74]—to consider either sensorimotor symptoms or cognitive symptoms as treatment targets and investigate the effects on both domains after pharmacological and non-pharmacological (stimulation techniques) interventions.
Strengths and limitations
The study sample size, a solid theoretical framework, and the use of two network indices, such as EI and strength centrality, are the main strengths of this study. However, this study also has limitations: First, sample size of BD and MDD subgroups was limited, which could imply that our results may have been more influenced by our SSD subgroup. Due to small group sizes in our MOD participants, we were not able to perform the network analyses in this subgroup, thus uncertainty remains whether our findings truly extend to MOD. Yet, in separate analyses including only SSD participants (n = 174), the results remained comparable. Still, the imbalance in the examined groups concerning the diagnosis could lead to sampling bias, making it difficult to generalize the findings to the broader population. Therefore, the question as to whether sensorimotor symptoms are really a transdiagnostic therapeutic target needs to be examined in future studies including larger and more balanced diagnostic groups. Second, by employing cross-sectional data, we cannot make inferences about long-term relationships between psychopathology, sensorimotor, and cognitive symptoms and we advocate longitudinal studies which could better test for causality. Third, we did not employ second-generation negative symptom scales such as the BNSS. However, our focus in this project was to analyze the entire psychopathological domain, for which PANSS remains the most widely used scale in SSD and other psychiatric disorders such as MOD (please refer to PANSS general symptoms). Fourth, we are aware of the previous network analyses [14, 15, 75, 76] which examined data on several levels ranging from total scores to sub-scores to single items level. The authors stated that more knowledge could be accumulated through the multilevel analysis. The study by Fried et al. [77] corroborate this view and performed network analyses on several levels. The authors [77] reported that relationships between depression and inflammation were strongly attenuated after controlling for BMI. They also concluded that decomposing sum scores may lead to reduced reliability [77]. Therefore, we assume that our network analysis could reveal more detailed insights if performed on several levels. However, in our study, we were interested in the interplay between psychopathology, sensorimotor, cognitive and global functioning more globally. Thus, we opted for subscores/subscales rather than single-item level scores. In addition, investigating many individual features (i.e., including more nodes in the network) makes network estimation more complex, requiring larger study groups in order to yield stable networks and consequently, we refrained from these analyses. Last but not least, our hypotheses were focused on investigations of the relationships between the different domains and not individual items.
Conclusion
This is the first study that sought to investigate the pattern of connections among a wide array of functional domains, such psychopathological symptoms, sensorimotor, cognitive, and global functioning in a transdiagnostic sample consisting SSD and MOD patients. Aspects of the pathophysiology underlying both SSD and MOD are likely shared, given the close relationship between NSS and cognitive functioning and the high centrality of sensorimotor symptoms. However, given that a significant proportion of the study participants had SSD, further research with more balanced diagnostic groups is required to determine whether sensorimotor symptoms truly represent a transdiagnostic therapeutic target.
Data availability
The data presented in this study are available from the corresponding author on reasonable request.
Change history
13 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00406-024-01824-w
References
Lange SMM, Schirmbeck F, Stek ML, Murk Jansen YR, van Rooijen G, de Haan L, Penninx B, Rhebergen D, Investigators G (2021) A comparison of depressive symptom profiles between current major depressive disorder and schizophrenia spectrum disorder. J Psychiatr Res 135:143–151
Kanchanatawan B, Sirivichayakul S, Thika S, Ruxrungtham K, Carvalho AF, Geffard M, Anderson G, Noto C, Ivanova R, Maes M (2017) Physio-somatic symptoms in schizophrenia: association with depression, anxiety, neurocognitive deficits and the tryptophan catabolite pathway. Metab Brain Dis 32:1003–1016
Fritze S, Sambataro F, Kubera KM, Brandt GA, Meyer-Lindenberg A, Wolf RC, Hirjak D (2022) Characterizing the sensorimotor domain in schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci 272:1097–1108
Arias JA, Williams C, Raghvani R, Aghajani M, Baez S, Belzung C, Booij L, Busatto G, Chiarella J, Fu CH, Ibanez A, Liddell BJ, Lowe L, Penninx B, Rosa P, Kemp AH (2020) The neuroscience of sadness: a multidisciplinary synthesis and collaborative review. Neurosci Biobehav Rev 111:199–228
Kovacs LN, Takacs ZK, Toth Z, Simon E, Schmelowszky A, Kokonyei G (2020) Rumination in major depressive and bipolar disorder—a meta-analysis. J Affect Disord 276:1131–1141
Chen L, Wang Q, Xu T (2023) Working memory function in patients with major depression disorder: a narrative review. Clin Psychol Psychother 30:281–293
van Eeden WA, van Hemert AM, Carlier IVE, Penninx BW, Spinhoven P, Giltay EJ (2019) Neuroticism and chronicity as predictors of 9-year course of individual depressive symptoms. J Affect Disord 252:484–492
Oliva V, Fanelli G, Kasper S, Zohar J, Souery D, Montgomery S, Albani D, Forloni G, Ferentinos P, Rujescu D, Mendlewicz J, Kas MJ, De Ronchi D, Fabbri C, Serretti A (2022) Social withdrawal as a trans-diagnostic predictor of short-term remission: a meta-analysis of five clinical cohorts. Int Clin Psychopharmacol 37:38–45
Song XM, Hu XW, Li Z, Gao Y, Ju X, Liu DY, Wang QN, Xue C, Cai YC, Bai R, Tan ZL, Northoff G (2021) Reduction of higher-order occipital gaba and impaired visual perception in acute major depressive disorder. Mol Psychiatry 26:6747–6755
Walther S, Bernard JA, Mittal VA, Shankman SA (2019) The utility of an rdoc motor domain to understand psychomotor symptoms in depression. Psychol Med 49:212–216
Fonseca-Pedrero E (2017) Network analysis: a new way of understanding psychopathology? Rev Psiquiatr Salud Ment 10:206–215
Jones PJ, Heeren A, McNally RJ (2017) Commentary: a network theory of mental disorders. Front Psychol 8:1305
Borsboom D (2017) A network theory of mental disorders. World Psychiatry 16:5–13
Abplanalp SJ, Braff DL, Light GA, Nuechterlein KH, Green MF, Consortium on the Genetics of S (2022) Understanding connections and boundaries between positive symptoms, negative symptoms, and role functioning among individuals with schizophrenia: a network psychometric approach. JAMA Psychiat 79:1014–1022
Abplanalp SJ, Mueser KT, Fulford D (2022) The centrality of motivation in psychosocial functioning: network and bifactor analysis of the quality of life scale in first-episode psychosis. Psychol Assess 34:205–216
Dal Santo F, Fonseca-Pedrero E, Garcia-Portilla MP, Gonzalez-Blanco L, Saiz PA, Galderisi S, Giordano GM, Bobes J (2022) Searching for bridges between psychopathology and real-world functioning in first-episode psychosis: a network analysis from the optimise trial. Eur Psychiatry 65:e33
Strauss GP, Esfahlani FZ, Galderisi S, Mucci A, Rossi A, Bucci P, Rocca P, Maj M, Kirkpatrick B, Ruiz I, Sayama H (2019) Network analysis reveals the latent structure of negative symptoms in schizophrenia. Schizophr Bull 45:1033–1041
Galderisi S, Rucci P, Kirkpatrick B, Mucci A, Gibertoni D, Rocca P, Rossi A, Bertolino A, Strauss GP, Aguglia E, Bellomo A, Murri MB, Bucci P, Carpiniello B, Comparelli A, Cuomo A, De Berardis D, Dell’Osso L, Di Fabio F, Gelao B, Marchesi C, Monteleone P, Montemagni C, Orsenigo G, Pacitti F, Roncone R, Santonastaso P, Siracusano A, Vignapiano A, Vita A, Zeppegno P, Maj M, Italian Network for Research on P (2018) Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: a network analysis. JAMA Psychiat 75:396–404
Sambataro F, Fritze S, Rashidi M, Topor CE, Kubera KM, Wolf RC, Hirjak D (2020) Moving forward: distinct sensorimotor abnormalities predict clinical outcome after 6 months in patients with schizophrenia. Eur Neuropsychopharmacol 36:72–82
Herold CJ, Duval CZ, Schröder J (2021) Neurological soft signs and cognition in the late course of chronic schizophrenia: a longitudinal study. Eur Arch Psychiatry Clin Neurosci 271:1465–1473
Bachmann S, Schröder J (2017) Neurological soft signs in schizophrenia: an update on the state- versus trait-perspective. Front Psych 8:272
Valerio MP, Lomastro J, Igoa A, Martino DJ (2023) Correlates of neurological soft signs in bipolar disorder. Eur Arch Psychiatry Clin Neurosci 273:1183–1190
Schröder J, Niethammer R, Geider FJ, Reitz C, Binkert M, Jauss M, Sauer H (1991) Neurological soft signs in schizophrenia. Schizophr Res 6:25–30
Hurford IM, Marder SR, Keefe RS, Reise SP, Bilder RM (2011) A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull 37:538–545
Zhao Q, Li Z, Huang J, Yan C, Dazzan P, Pantelis C, Cheung EF, Lui SS, Chan RC (2014) Neurological soft signs are not “soft” in brain structure and functional networks: evidence from ale meta-analysis. Schizophr Bull 40:626–641
Walther S, van Harten PN, Waddington JL, Cuesta MJ, Peralta V, Dupin L, Foucher JR, Sambataro F, Morrens M, Kubera KM, Pieters LE, Stegmayer K, Strik W, Wolf RC, Hirjak D (2020) Movement disorder and sensorimotor abnormalities in schizophrenia and other psychoses—European consensus on assessment and perspectives. Eur Neuropsychopharmacol 38:25–39
Pavlidou A, Walther S (2020) Using virtual reality as a tool in the rehabilitation of movement abnormalities in schizophrenia. Front Psychol 11:607312
Sass H, Wittchen HU, Zaudig M, IH (2003) Diagnostisches und statistisches Manual psychischer Störungen DSM-IV-TR: Textrevision. Hogrefe Verlag; Auflage: 1 (1. Januar 2003)
Hirjak D, Kubera KM, Northoff G, Fritze S, Bertolino AL, Topor CE, Schmitgen MM, Wolf RC (2019) Cortical contributions to distinct symptom dimensions of catatonia. Schizophr Bull 45:1184–1194
Hirjak D, Rashidi M, Kubera KM, Northoff G, Fritze S, Schmitgen MM, Sambataro F, Calhoun VD, Wolf RC (2019) Multimodal magnetic resonance imaging data fusion reveals distinct patterns of abnormal brain structure and function in catatonia. Schizophrenia Bull 46:202–210
Otte ML, Schmitgen MM, Kubera KM, Wolf ND, Fritze S, Geiger LS, Tost H, Seidl UW, Meyer-Lindenberg A, Hirjak D, Wolf RC (2022) Cortical morphology and illness insight in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 272(6):985–995. https://doi.org/10.1007/s00406-021-01328-x
Sambataro F, Hirjak D, Fritze S, Kubera KM, Northoff G, Calhoun VD, Meyer-Lindenberg A, Wolf RC (2021) Intrinsic neural network dynamics in catatonia. Hum Brain Mapp 42(18):6087–6098. https://doi.org/10.1002/hbm.25671
Margraf J, Schneider S, Ehlers A, Psychologie C-D-SFK (1994) Diagnostisches interview bei psychischen störungen: dips. Springer
Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, Geddes J, Davis JM (2015) Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull 41:1397–1402
Hayasaka Y, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, Barbui C, Leucht S, Furukawa TA (2015) Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord 180:179–184
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (panss) for schizophrenia. Schizophr Bull 13:261–276
Dsm-iiiR (1989) Dkudddusmpsr gaf-skala: global assessment of functioning scale. Beltz, Weinheim, Basel
Amieva H, Meillon C, Proust-Lima C, Dartigues JF (2019) Is low psychomotor speed a marker of brain vulnerability in late life? Digit symbol substitution test in the prediction of Alzheimer, Parkinson, stroke, disability, and depression. Dement Geriatr Cogn Disord 47:297–305
Boylu ME, Turan S, Guler EM, Boylu FB, Kilic O, Kocyigit A, Kirpinar I (2023) Changes in neuroactive steroids, neurotrophins and immunological biomarkers after monotherapy 8-week rtms treatment and their relationship with neurocognitive functions in depression. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-023-01704-9
Nixon N, Guo B, Kaylor-Hughes C, Simpson S, Garland A, Dalgleish T, Morriss R (2024) Specialist treatment for persistent depression in secondary care: sustained effects from a multicentre UK study at 24 and 36 months. J Affect Disord 345:70–77
R Core Team (2021) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org/
Karyakina M, Shmukler A (2021) Network analysis of cognitive deficit in patients with schizophrenia spectrum disorders. Schizophr Res Cogn 26:100213
Strauss GP, Esfahlani FZ, Kirkpatrick B, Allen DN, Gold JM, Visser KF, Sayama H (2019) Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr Bull 45:1319–1330
Strauss GP, Zamani Esfahlani F, Sayama H, Kirkpatrick B, Opler MG, Saoud JB, Davidson M, Luthringer R (2020) Network analysis indicates that avolition is the most central domain for the successful treatment of negative symptoms: evidence from the roluperidone randomized clinical trial. Schizophr Bull 46:964–970
Costantini G, Epskamp S, Borsboom D, Perugini M, Mõttus R, Waldorp LJ, Cramer AO (2015) State of the art personality research: a tutorial on network analysis of personality data in R. J Res Pers 54:13–29
Hevey D (2018) Network analysis: a brief overview and tutorial. Health Psychol Behav Med 6:301–328
Epskamp S, Borsboom D, Fried EI (2018) Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods 50:195–212
Epskamp S, Fried EI (2018) A tutorial on regularized partial correlation networks. Psychol Methods 23:617–634
Christensen AP, Golino H (2021) Estimating the stability of psychological dimensions via bootstrap exploratory graph analysis: a Monte Carlo simulation and tutorial. Psych 3:479–500
Golino H, Shi D, Christensen AP (2020) Investigating the performance of exploratory graph analysis and traditional techniques to identify the number of latent factors: a simulation and tutorial. Psychol Methods 25:292–320
Golino HF, Epskamp S (2017) Exploratory graph analysis: a new approach for estimating the number of dimensions in psychological research. PLoS ONE 12:e0174035
Cuesta MJ, Sánchez-Torres AM, de Jalón EG, Campos MS, Ibáñez B, Moreno-Izco L, Peralta V (2014) Spontaneous parkinsonism is associated with cognitive impairment in antipsychotic-naive patients with first-episode psychosis: a 6-month follow-up study. Schizophr Bull 40:1164–1173
Peralta V, Basterra V, Campos MS, de Jalón EG, Moreno-Izco L, Cuesta MJ (2012) Characterization of spontaneous Parkinsonism in drug-naive patients with non-affective psychotic disorders. Eur Arch Psychiatry Clin Neurosci 262:131–138
Wolf RC, Rashidi M, Schmitgen MM, Fritze S, Sambataro F, Kubera KM, Hirjak D (2021) Neurological soft signs predict auditory verbal hallucinations in patients with schizophrenia. Schizophr Bull 47:433–443
Osborne KJ, Walther S, Shankman SA, Mittal VA (2020) Psychomotor slowing in schizophrenia: implications for endophenotype and biomarker development. Biomark Neuropsychiatry 2:100016
Gay O, Plaze M, Oppenheim C, Gaillard R, Olie JP, Krebs MO, Cachia A (2016) Cognitive control deficit in patients with first-episode schizophrenia is associated with complex deviations of early brain development. J Psychiatry Neurosci 41:150267
Hirjak D, Meyer-Lindenberg A, Kubera KM, Thomann PA, Wolf RC (2018) Motor dysfunction as research domain in the period preceding manifest schizophrenia: a systematic review. Neurosci Biobehav Rev 87:87–105
Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC (2015) Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res 169:217–233
Zhao Q, Li Z, Huang J, Yan C, Dazzan P, Pantelis C, Cheung EF, Lui SS, Chan RC (2013) Neurological soft signs are not “soft” in brain structure and functional networks: evidence from ale meta-analysis. Schizophr Bull 40:626–641
Rusch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G (2007) Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res 93:79–89
Owen MJ, Sawa A, Mortensen PB (2016) Schizophrenia. Lancet (Lond, Engl) 388:86–97
Morrens M, Hulstijn W, Van Hecke J, Peuskens J, Sabbe BG (2006) Sensorimotor and cognitive slowing in schizophrenia as measured by the symbol digit substitution test. J Psychiatr Res 40:200–206
Velasques B, Machado S, Paes F, Cunha M, Sanfim A, Budde H, Cagy M, Anghinah R, Basile LF, Piedade R, Ribeiro P (2011) Sensorimotor integration and psychopathology: motor control abnormalities related to psychiatric disorders. World J Biol Psychiatry 12(8):560–573. https://doi.org/10.3109/15622975.2010.551405
Northoff G (2022) Spatiotemporal psychopathology - a novel approach to brain and symptoms. Noro Psikiyatr Ars 59(Suppl 1):S3–S9. https://doi.org/10.29399/npa.28146
Kharawala S, Hastedt C, Podhorna J, Shukla H, Kappelhoff B, Harvey PD (2022) The relationship between cognition and functioning in schizophrenia: a semi-systematic review. Schizophr Res Cogn 27:100217
Hirjak D, Wolf RC, Paternoga I, Kubera KM, Thomann AK, Stieltjes B, Maier-Hein KH, Thomann PA (2016) Neuroanatomical markers of neurological soft signs in recent-onset schizophrenia and asperger-syndrome. Brain Topogr 29(3):382–394. https://doi.org/10.1007/s10548-015-0468-9
Hirjak D, Kubera KM, Wolf RC, Thomann AK, Hell SK, Seidl U, Thomann PA (2015) Local brain gyrification as a marker of neurological soft signs in schizophrenia. Behav Brain Res 292:19–25. https://doi.org/10.1016/j.bbr.2015.05.048
Hirjak D, Rashidi M, Fritze S, Bertolino AL, Geiger LS, Zang Z, Kubera KM, Schmitgen MM, Sambataro F, Calhoun VD, Weisbrod M, Tost H, Wolf RC (2019) Patterns of co-altered brain structure and function underlying neurological soft signs in schizophrenia spectrum disorders. Hum Brain Mapp 40(17):5029–5041. https://doi.org/10.1002/hbm.24755
Fritze S, Bertolino AL, Kubera KM, Topor CE, Schmitgen MM, Wolf RC, Hirjak D (2019) Differential contributions of brainstem structures to neurological soft signs in first- and multiple-episode schizophrenia spectrum disorders. Schizophr Res 210:101–106. https://doi.org/10.1016/j.schres.2019.05.041
Borsboom D, Deserno MK, Rhemtulla M, Epskamp S, Fried EI, McNally RJ, Robinaugh DJ, Perugini M, Dalege J, Costantini G (2021) Network analysis of multivariate data in psychological science. Nat Rev Methods Primers 1:58
McNally RJ (2016) Can network analysis transform psychopathology? Behav Res Ther 86:95–104
McNally RJ (2019) The network takeover reaches psychopathology. Behav Brain Sci 42:e15
Gay O, Plaze M, Oppenheim C, Gaillard R, Olie JP, Krebs MO, Cachia A (2017) Cognitive control deficit in patients with first-episode schizophrenia is associated with complex deviations of early brain development. J Psychiatry Neurosci 42:87–94
Elliott H, Jones PJ, Schmidt U (2020) Central symptoms predict posttreatment outcomes and clinical impairment in anorexia nervosa: a network analysis. Clin Psychol Sci 8:139–154
Abplanalp SJ, Lee J, Horan WP, Kern RS, Penn DL, Green MF (2023) A bayesian network approach to social and nonsocial cognition in schizophrenia: are some domains more fundamental than others? Schizophr Bull 49:997–1006
Abplanalp SJ, Green MF (2022) Symptom structure in schizophrenia: implications of latent variable modeling vs network analysis. Schizophr Bull 48:538–543
Fried EI, von Stockert S, Haslbeck JMB, Lamers F, Schoevers RA, Penninx B (2020) Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychol Med 50:2682–2690
Acknowledgements
We are grateful to all the patients and their families for their time and interest in our studies.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Research Foundation (DFG, grant number DFG HI 1928/2-1, HI 1928/5-1, and HI 1928/6-1 to D.H.). The DFG had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
SF, GAB, and DH designed and organized the research. SF, GAB, JD, MK, SV, and JK collected the psychopathological data. SF, GAB, KMK, and DH accessed and verified the data. SF, SV, and DH analyzed the data. SF and DH wrote the first draft of the manuscript. All authors discussed the results, contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest in relation to the subject of this study.
Additional information
The original online version of this article was revised due to change in author affiliation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fritze, S., Brandt, G.A., Volkmer, S. et al. Deciphering the interplay between psychopathological symptoms, sensorimotor, cognitive and global functioning: a transdiagnostic network analysis. Eur Arch Psychiatry Clin Neurosci (2024). https://doi.org/10.1007/s00406-024-01782-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-024-01782-3