Abstract
Electroconvulsive therapy (ECT) is commonly used to treat treatment-resistant depression (TRD). However, our knowledge of the ECT-induced molecular mechanisms causing clinical improvement is limited. To address this issue, we developed the single-center, prospective observational DetECT study (“Multimodal Biomarkers of ECT in TRD”; registered 18/07/2022, www.clinicalTrials.gov, NCT05463562). Its objective is to identify molecular, psychological, socioeconomic, and clinical biomarkers of ECT response in TRD. We aim to recruit n = 134 patients in 3 years. Over the course of 12 biweekly ECT sessions (± 7 weeks), participant blood is collected before and 1 h after the first and seventh ECT and within 1 week after the twelfth session. In pilot subjects (first n = 10), additional blood draws are performed 3 and 6 h after the first ECT session to determine the optimal post-ECT blood draw interval. In blood samples, multiomic analyses are performed focusing on genotyping, epigenetics, RNA sequencing, neuron-derived exosomes, purines, and immunometabolics. To determine clinical response and side effects, participants are asked weekly to complete four standardized self-rating questionnaires on depressive and somatic symptoms. Additionally, clinician ratings are obtained three times (weeks 1, 4, and 7) within structured clinical interviews. Medical and sociodemographic data are extracted from patient records. The multimodal data collected are used to perform the conventional statistics as well as mixed linear modeling to identify clusters that link biobehavioural measures to ECT response. The DetECT study can provide important insight into the complex mechanisms of ECT in TRD and a step toward biologically informed and data-driven-based ECT biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is among the most prevalent disorders worldwide [1, 2]. Its 12-month prevalence is ~ 11% and women are affected twice as often as men [3, 4]. It is associated with high personal and socioeconomic costs, also through as well as an elevated morbidity compared to the general public [5,6,7]. In addition, patients with MDD experience higher cardiovascular and metabolic comorbidities, which contribute to the observed excess mortality [6, 7]. MDD is an episodic disorder and recurrence affects 40–50% [8]. A chronic course of disease (> 24 months) is found in about 25% of MDD subjects [9]. While a number of effective treatments exist, a high proportion of MDD patients do not sufficiently respond to current therapies. Treatment resistance (treatment-resistant depression, TRD) affects about 30% of even patients and is accompanied by higher rates of psychiatric and somatic morbidity as well as mortality [10,11,12]. TRD definitions are somewhat heterogeneous [13,14,15], yet the most common one is a lack of clinically meaningful improvement (non-response) following two antidepressant drugs administered in an appropriate dosage and duration [13, 16,17,18]. This definition is shared by the U.S. Food and Drug Administration (FDA) [19] as well as the European Medicines Agency [20] and the recent German federal depression treatment guidelines [21]. TRD is an important medical and societal issue [22], especially taking the globally rising incidence into account [23,24,25]. One in three TRD patients attempts suicide at least once in their lifetime, which is twice as high as in treatment-responsive depression [26]. TRD patients use health services more frequently and generate high healthcare costs, both at the absolute level as well as proportionally to the overall costs related to depression [27,28,29].
Beyond conventional pharmaco- and psychotherapy, neurostimulation techniques such as electroconvulsive therapy (ECT) are widely available, safe, and powerful treatment options in psychiatry [30,31,32,33]. About 80% of all ECTs are performed in patients with MDD [34, 35]. Though ECT should always be preceded by an individual risk–benefit assessment [36], studies and meta-analyses overall report markedly favorable response rates as high as 79% and remission in up to 70% of cases [37,38,39]. In severe and psychotic depressive episodes, Petrides et al. have even reported remission in 95% of their participants [40]. ECT is also a highly effective treatment option for TRD [37], with an efficacy comparable to the general response rates seen in treatment-responsive depression [41]. Studies and meta-analyses indicate an ECT-related response in 56–85% and a remission in about 48% of patients with prior medication failure or TRD, respectively [27, 41,42,43,44]. This response rate in TRD is similar to the response rates reported for antidepressant drugs and psychotherapy overall in non TRD patients of about 50–60% [45,46,47,48,49,50,51]. It is thus not surprising that the mean effect size of ECT (d ≈ 0.9–1.3) is higher than the one of antidepressants (d ≈ 0.37) or psychotherapy (d ≈ 0.75) [52,53,54,55,56,57,58]. ECT-induced remission is commonly achieved early during treatment (~ 3 weeks after ECT start) [27, 59]. ECT is also known to significantly reduce the risk of suicide [33, 60,61,62]. Due to the high recurrence rates of up to 81% after acute-phase ECT, maintenance sessions are recommended and performed in gradually decreasing frequency to preserve the initial therapy success [36, 39, 63,64,65,66,67,68]. During an ECT series, sub-/acute side effects, such as headaches, muscle soreness, nausea, transient hypertension, anterograde and retrograde amnesia (especially ± 3 days around the ECT), and postictal confusion, rarely a delirium, can occur due to the applied current and induced seizure apart from general anesthesia risks [69, 70]. These adverse effects are reversible and short-lived in the majority of cases (< 1 h post-ECT) [69]. The frequency of cognitive or memory side effects is associated with patient and disease characteristics as well as the ECT stimulation paradigm and intensity (e.g., strong, bitemporal stimulation over a long period of time: increased memory disturbance) [70,71,72,73]. On average, however, cognitive performance tends to remain the same or improve after ECT treatment [71, 74, 75]. Both magnetic resonance imaging and autopsy studies as well as animal experiments have not demonstrated any organic brain damage due to ECT [76,77,78,79,80]. The balance between strong clinical effects and a tolerable side effect profile makes ECT the gold standard of stimulation methods, provided appropriate patient selection [55, 75, 81, 82].
The therapeutic effect of ECT is thought to come from a combination of short- and long-term effects of the induced epileptic seizures [83]. There is evidence from both preclinical as well as clinical studies on possible molecular/biological mechanisms of action, but not always consistent across studies. The most important findings and hypotheses, however, can be summarized as follows: increase in neurotrophic factors such as brain-derived neurotrophic factors (BDNF) and enhanced neuroplasticity including the formation of new synapses, modulation of neurotransmitter metabolism and subsequent alterations in specific neurotransmission accompanied by modification of epigenetic processes and gene expression changes. Alterations in immune cell composition and cytokine levels and regulation and normalization of hypothalamic–pituitary–adrenal axis dysregulation have been reported. From neuroimaging studies, changes in gray and white matter volumes and normalization of altered functional connectivity are reported [27, 69, 83,84,85]. To date, however, it is not clear which of these biomolecular processes are relevant for the therapeutic success of ECT [86, 87]. Accordingly, no reliable and clinically applicable biomarkers for ECT are available for MDD and TRD [82, 88,89,90,91,92]. Due to this lack of objective biological measures, clinical decision on ECT is still solely informed by consensus-based treatment guidelines [36, 93, 94]. Similarly, prediction of ECT success is loosely based on a few clinical parameters like episode duration and severity [95, 96], failed prior pharmacotherapy [42, 43], age [97, 98], psychotic features [40], and early symptom improvement [99]. In order to improve clinical decision making and identify MDD patients with an optimal ECT risk–benefit profile, reliable biomarkers and multimodal prognosis models are urgently needed [40, 75]. Although a handful of studies have suggested potential biomarkers [100,101,102,103,104,105,106,107,108,109,110,111,112,113], so far none has proven valid or reliable enough for routine application in day-to-day care.
We have initiated the DetECT study to improve our understanding of the molecular mechanisms contributing to response to ECT in TRD and to discover novel biomarkers for clinical application. Our study relies on a multilevel data structure and multiomics approach including novel potential biomarkers such as: (i) neural cell-derived exosomes, as they hold the potential to serve as peripheral biomarkers mirroring ECT-induced molecular changes in the brain [114,115,116,117]; and (ii) purinergic signaling dynamics, potentially modulating immune mechanisms linked to treatment resistance and ECT response [118,119,120,121]. Our multidimensional data collection is described below and acknowledges the inherent complexity of MDD and the subsequent biobehavioral changes and patterns evoked by ECT. Ultimately, future biomarkers will likely be a cluster of molecular, behavioral, and clinical measures rather than one single parameter [70, 99].
Methods
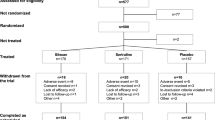
The DetECT study was initiated at the Max Planck Institute of Psychiatry’s (MPIP) research clinic in 2022. All study documents have been approved by the local ethics committee of the Ludwig-Maximilians-University Munich (Project registration number: 21–1087); see also https://www.psych.mpg.de/detect. The study is enrolled with the U.S. National Institute of Health (NIH) clinical study register www.ClinicalTrials.gov (Study ID: NCT05463562, registration date 18/07/2022). The project is conducted in strict accordance with all relevant national and international regulations including the Declaration of Helsinki. A detailed schematic of the study design can be found in Fig. 1.
Overview of serial blood draws and psychometry in the DetECT study. In the study’s main phase, blood draws are performed before and after the first and seventh as well as within 1 week after the twelfth ECT session. To estimate clinical response and side effects over time, participants are asked weekly to complete four standardized self-rating questionnaires on depressive and somatic symptoms (Patient Health Questionnaires 9 and 15, Questionnaire on Mental Capacity, Beck Depression Inventory II). Foreign ratings are obtained thrice by a standardized clinical interview (Montgomery-Åsberg Depression Rating Scale, Hamilton Rating Scale for Depression, Global Assessment of Functioning) (created with www.Biorender.com)
Study design and population
The DetECT project has a prospective and exploratory design. Recruitment is planned to last 3 years. The study is conducted exclusively in adult (≥ 18 years) and legally competent severely depressed in-patients receiving treatment at the MPIP’s research clinic in the form of ECT. The study design is purely observational. Therefore, both the diagnostic and treatment process including the mode of ECT administration as well as pharmacotherapy is determined solely by the attending psychiatrist in joint decision with the patient based on the overall clinical situation in accordance with the German depression treatment guidelines [21]. Diagnosis is made by a trained psychiatrist in compliance with the depression criteria of the International Classification of Diseases 10th revision (ICD-10). Patients included in the study suffer from TRD that is defined in accordance with the FDA and EMA guidelines, i.e., two or more failed antidepressant treatments in adequate dosing and duration. Depression severity is evaluated via standardized self- and clinician ratings, as described later in detail. Inclusion and exclusion criteria are listed in Table 1.
In study participants, serial venous blood draws (prior fasting: ≥ 6 h) are performed before and after the first and seventh as well as after the twelfth ECT session. A detailed schematic of the study design is given in Fig. 1. Pre-ECT blood draws with a total volume of 36 ml include collection of ethylenediamine tetra acetic acid (EDTA) (Sarstedt, S-Monovette® K3 EDTA) and serum (Sarstedt, S-Monovette® Serum Gel) tubes as well as PaxGene Blood RNA Tubes (Qiagen) for the extraction and analysis of DNA and epigenetic measures (e.g., methylation patterns), RNA including messenger and micro-RNA (mRNA and miRNA, respectively), neuron-derived exosomes, purines, hormones, proteins, electrolytes, metabolic parameters, inflammatory markers, and peripheral blood mononuclear cells (PBMCs). Post-ECT blood draws include only EDTA and serum tubes (Sarstedt) for total of 10 ml. In a subset of n = 10 participants, which are recruited at the very beginning of the study’s field phase (DetECT pilot phase), the ECT blood collection after the first ECT consists of three time points (1 h, 3 h, and 6 h post). The rationale for this schedule is to characterize the temporal post-ECT dynamics. This would also allow to discriminate between an immediate ECT-induced release of brain-derived molecules and cells through the blood–brain barrier (fast pathway) or a delayed release via cerebrospinal fluid drainage (slow pathway). To achieve this, the pilot phase materials and data are analyzed with regards to the different multiomics read-outs to determine the optimal post-ECT time point for the study main phase. To reduce batch effects, samples are stored in the MPIP’s biobanking unit in freezers for later analyses with larger sample sizes.
In addition to the serial blood draws, participants are asked to answer the following self-rating questionnaires concerning their depressive and somatic symptoms on a weekly basis: Patient Health Questionnaires 9 and 15 (PHQ-9/-15) [122,123,124,125], the Questionnaire on Mental Capacity (FLei) [126], and the Beck Depression Inventory (BDI-II) [127,128,129]. The reference-interval of the self-rating questionnaires was customized from two (PHQ-9, BDI-II) and four (PHQ-15) weeks as well as six month (FLei) to the week prior to match the DetECT study design. Moreover, three clinician-rating instruments are assessed via a clinical interview in week 1, 4, and 7 of the study period: the Montgomery-Åsberg Depression Rating Scale (MADRS) [130, 131], the Hamilton Rating Scale for Depression with 21 items (HAM-D21) [132,133,134], and the DSM-IV derived Global Assessment of Functioning (GAF) [135, 136]. In addition to study related procedures, metabolic parameters (i.e., body mass index (BMI) or blood pressure (BP)) as well as medical information (e.g., patient and family anamnesis, past and recent medication including concomitant psychopharmacological treatment pre and during ECT allergies, drug use, psychiatric and somatic diagnoses) and sociodemographic data are extracted from patient records. See Fig. 1 for further detail.
Screening and recruitment
All patients who receive inpatient psychiatric treatment including a pending ECT for a confirmed TRD are informed about the DetECT study and the possibility of participating. This is done by the treating/responsible psychiatrist or another treatment team member. Rarely, first contact with the study team is made through an interested patient directly. If patients express an interest in participating, after receiving first and general information about the DetECT study, their eligibility and contact information is passed onto the MPIP Study Center. Study participation is completely voluntary. After obtaining the signed informed consent form, all study-related procedures are commenced.
Study timeline
The study period can be divided into four distinct consecutive work phases (see Fig. 2). Phase one commenced in September 2022 and will recruit minimum of the n = 10 pilot participants. It is estimated to last half a year. After completion, pilot materials and data are analyzed (Phase two). Here, measurements of highly dynamic parameters (e.g., RNA expression, neuron-derived exosome analyses, and immunological measures including cytokines and purines) are carried out and validated methodically. We expect this to yield meaningful and novel information about the post-ECT temporal dynamics of different biological read-outs. The focus of the laboratory and statistical analyses is at which time (i.e., either after 1 h, 3 h, or 6 h) potential biomarkers show the largest deflection from baseline. The timeframe for these analyses is expected to be half a year. Based on the biomarker dynamics of the pilot samples, the post-ECT blood collection interval for the DetECT main phase (Phase three) will be determined. In the main phase of the study, a total of n = 124 participants will be recruited over the course of 2 years. Finally (Phase four), the data obtained are analyzed and published. Here, the focus is not only on the creation of new scientific knowledge, but the clinical implementation of the identified biomarkers.
Schematic DetECT study timeline. The study period can be divided in four phases: (I) recruitment of the n = 10 pilot participants; (II) analyses of pilot materials and data. Primarily highly dynamic parameters (e.g., RNA expression, neuron-derived exosomes, immune cells, cytokines, and purines) are measured and validated. (III) Study main phase aiming to recruit a total of n = 124 participants over the course of 2 years. (IV) Finally, data are analyzed and published with a focus on the potential of the novel insights and biomarkers for clinical application (created with www.Biorender.com)
Longitudinal psychometric measures
To simultaneously measure depressive, neurocognitive, and somatic symptoms longitudinally in DetECT participants, we have selected four self-rating and three clinician-rating instruments. Since DetECT patients suffer from severe TRD and are thus heavily affected, psychometry was tailored to minimize participant efforts, while maximizing read-outs and item validation options post hoc. Only German questionnaire versions were used (see [126, 131, 137,138,139,140]).
Self-rating questionnaires: The PHQ-9 and PHQ-15 are both subscales of the patient health questionnaire (PHQ), which have proven effective for monitoring depression and somatic symptom burden [141]. The PHQ-9 is a self-administered depression screening, which contains a dimensional answer category (0 = not at all; 3 = nearly every day) for the nine depression criteria derived of the DSM-IV [123, 142]. The BDI-II is, similar to the PHQ-9, a multiple-choice self-report questionnaire with high validity and reliability [127,128,129, 143]. It is composed of 21 items, which have values of 0 to 3, to assess the presence as well as severity of depression [127]. The PHQ-15, on the other hand, is a self-rating questionnaire to assess somatic symptom load via 15 questions (0 = not bothered at all; 2 = bothered a lot). Summary scores are used as indicators of somatic symptom burden [125]. The FLei is a comparatively novel measure of neurocognitive function. By use of 35 questions with answers ranging from 0 = never to 4 = very frequently, the following domains are assessed: (I) attention, (II) memory, (III) executive functions, (IV) control scale [126]. Based on the FLei domain and sum scores, a longitudinal neurocognitive profile can be computed.
Clinician-rating questionnaires: Both, the MADRS and HAM-D21 are obtained by trained study personnel in a clinical interview. The MADRS (10-question instrument) and the HAM-D21 (21 items) both assess depression presence and severity [130,131,132,133,134]. The GAF is a functionality and severity score for psychiatric disorders ranging from 0 (= maximum symptom severity and load, extreme sociofunctional impairment) to 100 (= no symptoms or impairment). The index accounts for overall psychiatric symptom severity and sociofunctional impairment [135, 136].
Sample analytics: blood-based multiomics and biomarker screening
Using the collected and frozen within-subject blood samples, we aim to perform multiomics and deep phenotyping. To reduce batch effects and overall experimental variability, all laboratory measurements for a certain measure are performed together.
Genotyping and epigenetic analyses
Using DNA isolated with a conventional extraction kit from EDTA blood, genotyping and epigenetic profiling will be performed using array-based approaches. Quality is ensured due to our in-house standard operating procedure.
mRNA sequencing
Following mRNA extraction from thawed EDTA blood stored in PaxGene Blood RNA Tubes (Qiagen), we will use commercially available Library Prep Kits to generate sequencing libraries. Libraries will be multiplexed and sequenced on an Illumina® platform.
Neuron-derived exosomes and cargo analysis
Neuronal exosome isolation will be performed based on the protocol by Saeedi and Nagy et al. 2021 [144]. In brief, exosomes extracted from EDTA-plasma will be enriched using neuronal markers (e.g., synaptosomal-associated protein of 25 kDa, SNAP-25). Subsequently, exosome cargo will be analyzed focusing on miRNAs.
Cytokine and immune metabolite quantification
For cytokine quantifications, we plan to use custom-designed high-sensitivity immunomagnetic assays. Cytokine panels will be designed to reflect both pro- and anti-inflammatory states, in parts based on findings from large meta-analyses on cytokine changes in MDD [145]. Additionally, we will conduct ELISAs targeting other immunogenic metabolites like kynurenine and quinolinic acid [146].
Purine measurements
Total purine levels (mM) are measured immediately after venous blood collection in 40 µl of whole blood using high-sensitivity electrochemical sensors (Zimmerman & Peacock). Serum uric acid levels from routine blood draws at admission and comorbid gout and/or anti-gout medication is extracted from patient records and used as a covariable.
Immune cell phenotyping
To characterize immune profiles beyond cytokine clusters, we intend to perform both flow cytometry and fluorescence-activated cell sorting (FACS) using the acquired PBMCs. Based on changes reported in prior ECT studies [84, 147,148,149,150,151,152], we will use surface and intracellular markers to characterize immune cell subpopulations.
Metabolic and endocrine profiling
In addition to humoral and cellular immune profiling, we aim to characterize metabolic and endocrine states using established clinical and blood markers (e.g., BMI, BP, serum lipids, leptin and ghrelin, PBMC membrane proteins like CD220 or GLUT1, corticotropin-releasing hormone, and cortisol). This approach aligns with the growing evidence linking immunometabolic and neuroendocrine dysregulation to MDD [153,154,155,156,157,158].
Study hypotheses and objectives
The primary hypothesis of the DetECT study is that there exists a joint biological function and clinically meaningful connection between changes in gene expression and regulation, immunometabolic conditions, purinergic signaling, and CNS-derived exosome release in TRD patients receiving ECT. The secondary hypothesis is that these changes can be utilized to predict and monitor ECT outcome in TRD patients.
The main study objective is therefore to identify biological, psychological, socioeconomic, and clinical parameters (biomarkers) that provide detailed information about the general or individual factors of ECT success and the biobehavioral disease subgroups these changes are linked to. The DetECT data may also serve as a cornerstone for the development of a biologically founded, multimodal prognosis model for ECT use in TRD. As a subordinate objective, we hope to generate novel insight into the molecular effects of ECT in TRD using the generated omics data. Taken together, we believe that the DetECT results can aid precision medicine approaches for ECT application and to boost its social as well as clinical acceptance.
Power calculation
In complex psychiatric illnesses, such as MDD, which arise from the reciprocal interaction of endogenous and exogenous influences (interaction: genes x environment) [159], the effect of single parameters is small. Only the interaction of many and qualitatively heterogeneous components leads to the disease. The best-known explanatory model is the diathesis-stress model, in which the interaction of individual vulnerability (biological and psychosocial) and external influences is acknowledged. Therefore, and due to the exploratory and multiomics approach of the DetECT study focusing on unstudied parameters, sample size calculation cannot simply be performed based on preexisting study results. Accordingly, we have based our power calculation on the clinical ECT efficacy [55, 56], as it is a composite indicator of the interplay of multiple ECT-induced biological mechanisms and their effect sizes, respectively. Using G*Power 3 Software comparing responders with non-responders assuming a moderate effect size of d = 0.5, a significance level α = 0.05, and a power of 1–β = 0.8, we computed a sample size of n = 134 [160].
Prospective statistical analysis
The statistical analysis of the DetECT study mainly focuses on comparing ECT responders with non-responders (internal control group) within the sample. Clinical response indicated by overall and sub-domain changes in psychometry will serve as main dependent variables and analyses focus on within-subject changes over time. On the one hand, we aim to use variance and correlation analyses (e.g., repeated-measures ANOVA, Pearson’s r and correlation matrices), regression models (e.g., linear or logistic regression), and principal component analysis to first identify both significant main and interaction effects between biobehavioral parameters and treatment response. These analyses will be performed for all measures pre-, during, and post-treatment. Aside from using single parameters and the area under the curve as independent variables, we will employ index and score approaches (e.g., inflammatory indexing and Z scores) to allow combined analyses of functionally connected processes. Following these steps, we plan to employ linear mixed models (LMM) to examine if a single or parameter cluster is linked to remission, treatment response, failure, and side effects. As described above, we will also use single and combined as well as over time parameters, indices, or scores to perform LMM. Factors like the SES, comorbidities, smoking, concomitant pharmacological treatment (both general and psychiatry specific), and mode of ECT application (e.g., right-unilateral vs. left-anterior-right-temporal) will be used as covariates. Multiple comparisons correction will be applied. To further ensure that the identified associations are not only statistically significant, but also represent a relevant underlying biological mechanism, we will report both significance (p value) as well as effect size (e.g., Cohen’s d or comparable measures) in prospective DetECT publications, as recommended for instance by Goodman et al. 2019 [161].
Conclusion and outlook
The DetECT study is among the first trials to conduct multiomics-based biomarker screening using longitudinal within-subject blood collection and psychometry combined with clinical information in TRD subjects receiving ECT. The expected multilevel data structure will aid the generation of novel knowledge on both disease and therapy response mechanisms on a clinical, cellular, and molecular level. These insights will then enable a targeted search for biomarkers associated with beneficial outcomes and biobehavioral depression subtypes. Ideally, these findings will also be a first step toward a clinically applicable tool to predict ECT efficacy in TRD patients. The latter would ultimately pave the way toward personalization and specification of ECT application.
Availability of data and materials
The data generated and analyzed contain clinical data that cannot be made publicly available due to privacy rights of participants. In principle, data may be made available given a legitimate and science-related request.
References
World Health Organization, (2017) Depression and Other Common Mental Disorders: Global Health Estimates. Licence: CC BY-NC-SA 3.0 IGO
Spencer J (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858
Jacobi F et al (2014) Psychische Störungen in der Allgemeinbevölkerung. Nervenarzt 85(1):77–87
Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138
König H, König HH, Konnopka A (2019) The excess costs of depression: a systematic review and meta-analysis. Epidemiol Psychiatr Sci 29:e30
Steffen A et al (2020) Mental and somatic comorbidity of depression: a comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry 20(1):142
Kessler RC (2012) The costs of depression. Psychiatr Clin North Am 35(1):1–14
Nuggerud-Galeas S et al (2020) Analysis of depressive episodes, their recurrence and pharmacologic treatment in primary care patients: A retrospective descriptive study. PLoS ONE 15(5):e0233454
Bschor T, Bauer M, Adli M (2014) Chronic and treatment resistant depression: diagnosis and stepwise therapy. Dtsch Arztebl Int 111(45):766–775
Döme P et al (2021) Clinical characteristics of treatment-resistant depression in adults in Hungary: real-world evidence from a 7-year-long retrospective data analysis. PLoS ONE 16(1):e0245510
Nübel J et al (2020) Persistent depressive disorder across the adult lifespan: results from clinical and population-based surveys in Germany. BMC Psychiatry 20(1):58
Zhdanava M et al (2021) The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. https://doi.org/10.4088/JCP.20m13699
Brown S et al (2019) Current and common definitions of treatment-resistant depression: findings from a systematic review and qualitative interviews. Can J Psychiatry 64(6):380–387
Rybak YE et al (2021) Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety 38(4):456–467
Gaynes BN et al (2020) Defining treatment-resistant depression. Depress Anxiety 37(2):134–145
Bennabi D et al (2019) Clinical guidelines for the management of treatment-resistant depression: French recommendations from experts, the French Association for Biological Psychiatry and Neuropsychopharmacology and the fondation FondaMental. BMC Psychiatry 19(1):262
Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiat 53(8):649–659
Sforzini L et al (2022) A Delphi-method-based consensus guideline for definition of treatment-resistant depression for clinical trials. Mol Psychiatry 27(3):1286–1299
Administration FsD. (2018) Major Depressive Disorder: Developing Drugs for Treatment Guidance for Industry - DRAFT GUIDANCE, U.S.D.o.H.a.H.S.F.a.D.A.C.f.D.E.a.R. (CDER), Editor
European Medicines Agency (EMA) C.f.M.P.f.H.U.C. (2013) Guideline on clinical investigation of medicinal products in the treatment of depression
Bundesärztekammer KB (2022) Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, Nationale VersorgungsLeitlinie - Unipolare Depression [Langfassung]., ÄZQ – Redaktion Nationale VersorgungsLeitlinien. p. AWMF-Register-Nr. nvl-005.
Demyttenaere K, Van Duppen Z (2019) The impact of (the concept of) treatment-resistant depression: an opinion review. Int J Neuropsychopharmacol 22(2):85–92
Moreno-Agostino D et al (2021) Global trends in the prevalence and incidence of depression:a systematic review and meta-analysis. J Affect Disord 281:235–243
Santomauro DF et al (2021) Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398(10312):1700–1712
Racine N et al (2021) Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr 175(11):1142–1150
Bergfeld IO et al (2018) Treatment-resistant depression and suicidality. J Affect Disord 235:362–367
Subramanian S et al (2022) Electroconvulsive therapy in treatment resistant depression. J Neurol Sci 434:120095
Olchanski N et al (2013) The economic burden of treatment-resistant depression. Clin Ther 35(4):512–522
Mrazek DA et al (2014) A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv 65(8):977–987
Bundesärztekammer (2003) Stellungnahme zur Elektrokrampftherapie (EKT) als psychiatrische Behandlungsmaßnahme. Deutsches Ärzteblatt. 100(8)
Royal College of Psychiatrists (2017) Statement on electroconvulsive therapy (ECT)-position, statement CERT01/17
Jaffe R (2002) The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association. Am J Psychiatry 159(2):331–331
Liang CS et al (2017) In-hospital mortality among electroconvulsive therapy recipients: a 17-year nationwide population-based retrospective study. Eur Psychiatry 42:29–35
Loh N et al (2013) Accessibility, standards and challenges of electroconvulsive therapy in Western industrialized countries: a German example. World J Biol Psychiatry 14(6):432–440
Baghai TM, Möller A, H. J. and R. Rupprecht, (2005) Elektrokonvulsionstherapie an der Klinik für Psychiatrie und Psychotherapie der Universität München. Nervenarzt 76(5):597–612
Bauer M et al (2013) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 14(5):334–385
Husain MM et al (2004) Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 65(4):485–491
Bahji A et al (2019) ECT beyond unipolar major depression: systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr Scand 139(3):214–226
Brakemeier EL et al (2014) Cognitive-behavioral therapy as continuation treatment to sustain response after electroconvulsive therapy in depression: a randomized controlled trial. Biol Psychiatry 76(3):194–202
Petrides G et al (2001) ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ect 17(4):244–253
Husain SS et al (2004) Electroconvulsive therapy in depressive illness that has not responded to drug treatment. J Affect Disord 83(2–3):121–126
Heijnen WT et al (2010) Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol 30(5):616–619
Prudic J et al (1996) Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry 153(8):985–992
Tokutsu Y et al (2013) Follow-up study on electroconvulsive therapy in treatment-resistant depressed patients after remission: a chart review. Clin psychopharmacol Neurosci Off Sci J Korean Coll Neuropsychopharmacol 11(1):34–38
Levkovitz Y, Tedeschini E, Papakostas GI (2011) Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry 72(4):509–514
Plöderl M, Hengartner MP (2019) Guidelines for the pharmacological acute treatment of major depression: conflicts with current evidence as demonstrated with the German S3-guidelines. BMC Psychiatry 19(1):265
Cuijpers P, Stringaris A, Wolpert M (2020) Treatment outcomes for depression: challenges and opportunities. Lancet Psychiatry 7(11):925–927
Cuijpers P et al (2014) The effects of psychotherapies for major depression in adults on remission, recovery and improvement: a meta-analysis. J Affect Disord 159:118–126
Cuijpers P et al (2021) The effects of psychotherapies for depression on response, remission, reliable change, and deterioration: A meta-analysis. Acta Psychiatr Scand 144(3):288–299
Taliaz D et al (2021) Optimizing prediction of response to antidepressant medications using machine learning and integrated genetic, clinical, and demographic data. Transl Psychiatry 11(1):381
Khan A et al (2017) Has the rising placebo response impacted antidepressant clinical trial outcome? aata from the US Food and Drug Administration 1987–2013. World Psychiatry 16(2):181–192
Micallef-Trigona B (2014) Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat 2014:135049
Turner EH et al (2008) Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 358(3):252–260
Cuijpers P et al (2010) The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol Med 40(2):211–223
Kho KH et al (2003) A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT 19(3):139–147
Pagnin D et al (2004) Efficacy of ECT in depression a meta-analytic review. J ECT 20(1):13–20
Cipriani A et al (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet 391(10128):1357–1366
UK-ECT-Review-Group (2003) Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 361(9360):799–808
Kellner CH et al (2016) Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE Study. Am J Psychiatry 173(11):1101–1109
Oji C, Moore TA, Gutierrez CA (2015) A review of electroconvulsive therapy in suicidality. Mental Health Clin 5(5):212–215
Kellner CH et al (2005) Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry 162(5):977–982
Slade EP et al (2017) Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiat 74(8):798–804
Nordenskjöld A et al (2013) Continuation electroconvulsive therapy with pharmacotherapy versus pharmacotherapy alone for prevention of relapse of depression: a randomized controlled trial. J ect 29(2):86–92
Kellner CH, M.D., et al (2016) A novel strategy for continuation ECT in geriatric depression: phase 2 of the PRIDE study. Am J Psychiatry 173(11):1110–1118
Jelovac A, Kolshus E, McLoughlin DM (2013) Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology 38(12):2467–2474
Petrides G et al (2011) Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology 64(3):129–140
Bourgon LN, Kellner CH (2000) Relapse of depression after ECT: a review. J ect 16(1):19–31
Sackeim HA et al (2001) Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 285(10):1299–1307
Lisanby SH (2007) Electroconvulsive therapy for depression. N Engl J Med 357(19):1939–1945
Payne NA, Prudic J (2009) Electroconvulsive therapy: Part I a perspective on the evolution and current practice of ECT. J Psychiatric Pract. 15(5):346–368
Porter RJ et al (2020) Cognitive side-effects of electroconvulsive therapy: what are they, how to monitor them and what to tell patients. BJPsych Open 6(3):e40
Oremus C et al (2015) Effects of electroconvulsive therapy on cognitive functioning in patients with depression: protocol for a systematic review and meta-analysis. BMJ Open 5(3):e006966
Maria Semkovska PhD et al (2016) Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep) a pragmatic, randomized non-inferiority trial. Am J Psychiatry 173(4):408–417
Semkovska M, McLoughlin DM (2010) Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry 68(6):568–577
Luccarelli J et al (2020) Maintenance ECT is associated with sustained improvement in depression symptoms without adverse cognitive effects in a retrospective cohort of 100 patients each receiving 50 or more ECT treatments. J Affect Disord 271:109–114
Dwork AJ et al (2004) Absence of histological lesions in primate models of ECT and magnetic seizure therapy. Am J Psychiatry 161(3):576–578
Puri BK et al (1998) Does electroconvulsive therapy lead to changes in cerebral structure. Br J Psychiatry 173:267
Ende G et al (2000) The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry 57(10):937–943
Tosun Ş et al (2020) Proton magnetic resonance spectroscopic analysis of changes in brain metabolites following electroconvulsive therapy in patients with major depressive disorder. Int J Psychiatry Clin Pract 24(1):96–101
Obergriesser T et al (2003) Long-term follow-up of magnetic resonance-detectable choline signal changes in the hippocampus of patients treated with electroconvulsive therapy. J Clin Psychiatry 64(7):775–780
Kellner CH et al (2012) Appropriateness for electroconvulsive therapy (ECT) can be assessed on a three-item scale. Med Hypotheses 79(2):204–206
Kraus C et al (2019) Prognosis and improved outcomes in major depression: a review. Transl Psychiatry 9(1):127
Singh A, Kar SK (2017) How electroconvulsive therapy works?: understanding the neurobiological mechanisms. Clin Psychopharmacol Neurosci 15(3):210–221
van Buel EM et al (2015) Immune and neurotrophin stimulation by electroconvulsive therapy: is some inflammation needed after all? Transl Psychiatry 5(7):e609
Ousdal OT et al (2022) The neurobiological effects of electroconvulsive therapy studied through magnetic resonance: what have we learned, and where do we go? Biol Psychiatry 91(6):540–549
Voineskos D, Daskalakis ZJ, Blumberger DM (2020) Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat 16:221–234
Binder EB (2020) Understanding the mechanisms of treatment response in depression, focus on electro-convulsive therapy. Eur Arch Psychiatry Clin Neurosci 270(7):789–791
Chojnowska S et al (2021) Salivary biomarkers of stress, anxiety and depression. J Clin Med 10(3):517
Lopresti AL et al (2014) A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry 48:102–111
Mucci F et al (2020) State-of-the-art: inflammatory and metabolic markers in mood disorders. Life (Basel, Switzerland) 10(6):82
Nobis A, Zalewski D, Waszkiewicz N (2020) Peripheral markers of depression. J Clin Med 9(12):3793
Kennis M et al (2020) Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry 25(2):321–338
Deutsche Gesellschaft für Psychiatrie und Psychotherapie, Psychosomatik und Nervenheilkunde: Mitteilungen der DGPPN 7/2012. Nervenarzt 83(7):919–925
American Psychological Association (2009) Clinical practice guideline for the treatment of depression across three age cohorts. Guideline Development Panel for the Treatment of Depressive Disorders. https://www.apa.org/depression-guideline
Haq AU et al (2015) Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry 76(10):1374–1384
van Diermen L et al (2018) The Maudsley Staging Method as predictor of electroconvulsive therapy effectiveness in depression. Acta Psychiatr Scand 138(6):605–614
Rhebergen D et al (2015) Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry 23(3):274–282
van der Wurff FB et al (2003) The efficacy and safety of ECT in depressed older adults: a literature review. Int J Geriatr Psychiatry 18(10):894–904
Birkenhager TK, Roos J, Kamperman AM (2019) Improvement after two sessions of electroconvulsive therapy predicts final remission in in-patients with major depression. Acta Psychiatr Scand 140(3):189–195
van Waarde JA et al (2015) A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol Psychiatry 20(5):609–614
Cao B et al (2018) Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Sci Rep 8(1):5434
Nuninga JO et al (2020) Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry 25(7):1559–1568
Redlich R et al (2016) Prediction of individual response to electroconvulsive therapy via machine learning on structural magnetic resonance imaging data. JAMA Psychiat 73(6):557–564
Li M et al (2020) Effects of electroconvulsive therapy on depression and its potential mechanism. Front Psychol. https://doi.org/10.3389/fpsyg.2020.00080
Kruse JL et al (2018) Inflammation and improvement of depression following electroconvulsive therapy in treatment-resistant depression. J Clin Psychiatry. https://doi.org/10.4088/JCP.17m11597
Shibasaki C et al (2018) Possible Association between Serum Matrix Metalloproteinase-9 (MMP-9) levels and relapse in depressed patients following electroconvulsive therapy (ECT). Int J Neuropsychopharmacol 21(3):236–241
Foo JC et al (2019) Evidence for increased genetic risk load for major depression in patients assigned to electroconvulsive therapy. Am J Med Genet B Neuropsychiatr Genet 180(1):35–45
Kleimann A et al (2015) BDNF serum levels and promoter methylation of BDNF exon I, IV and VI in depressed patients receiving electroconvulsive therapy. J Neural Transm (Vienna) 122(6):925–928
Beamer E et al (2021) Elevated blood purine levels as a biomarker of seizures and epilepsy. Epilepsia 62(3):817–828
Belge JB et al (2021) Inflammatory markers may inform the effects of electroconvulsive therapy on cognition in patients with depression. Neuropsychobiology 80(6):493
Freire TFV, Rocha NSD, Fleck MPA (2017) The association of electroconvulsive therapy to pharmacological treatment and its influence on cytokines. J Psychiatr Res 92:205–211
Järventausta K et al (2017) Changes in interleukin-6 levels during electroconvulsive therapy may reflect the therapeutic response in major depression. Acta Psychiatr Scand 135(1):87–92
Gay F et al (2021) Cytokines changes associated with electroconvulsive therapy in patients with treatment-resistant depression: a Meta-analysis. Psychiatry Res 297:113735
Hornung S, Dutta S, Bitan G (2020) CNS-derived blood exosomes as a promising source of biomarkers: opportunities and challenges. Front Mol Neurosci 13:38
Wei Z-X et al (2020) Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology 45(6):1050–1058
Saeedi S et al (2019) The emerging role of exosomes in mental disorders. Transl Psychiatry. https://doi.org/10.1038/s41398-019-0459-9
Sharma P et al (2019) Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci 116(32):16086–16094
von Mücke-Heim I-A, Deussing JM (2022) The P2X7 receptor in mood disorders: Emerging target in immunopsychiatry, from bench to bedside. Neuropharmacology 224:109366
Sadek A-R, Knight GE, Burnstock G (2011) Electroconvulsive therapy: a novel hypothesis for the involvement of purinergic signalling. Purinergic Signal 7(4):447–452
Andreou B et al (2022) Longitudinal trajectory of response to electroconvulsive therapy associated with transient immune response & white matter alteration post-stimulation. Transl Psychiatry 12(1):191
Sepulveda-Rodriguez A et al (2019) Electroconvulsive shock enhances responsive motility and purinergic currents in microglia in the mouse hippocampus. eNeuro. https://doi.org/10.1523/ENEURO.0056-19.2019
Levis B, Benedetti A, Thombs B (2019) Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: Individual participant data meta-analysis. BMJ 365:l1476
Sun Y et al (2020) The reliability and validity of PHQ-9 in patients with major depressive disorder in psychiatric hospital. BMC Psychiatry 20(1):474–474
Kocalevent R-D, Hinz A, Brähler E (2013) Standardization of a screening instrument (PHQ-15) for somatization syndromes in the general population. BMC Psychiatry 13(1):91
Kroenke K, Spitzer R, Williams J (2002) The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 64:258–266
Beblo T et al (2010) Entwicklung eines Fragebogens zur subjektiven Einschätzung der geistigen Leistungsfähigkeit (FLei) bei Patienten mit psychischen Störungen. Z Neuropsychol 21(3):143–151
Upton J (2013) Beck Depression Inventory. In: Gellman MD, Turner JR (eds) Encyclopedia of Behavioral Medicine. Springer, NY, pp 178–179
Kuehner C et al (2007) Reliability and validity of the Revised Beck Depression Inventory (BDI-II): Results from German samples. Nervenarzt 78:651–656
Hautzinger M, K.F., Kühner C., BDI-II. Das Beck depressions-Inventar II. Revision. Manual:. 2006: Pearson
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Schmidtke A et al (1988) (1985) [Studies of the reliability and validity of the German version of the Montgomery-Asberg Depression Rating Scale (MADRS)]. Schweiz Arch Neurol Psychiatr 139(2):51–65
Zimmerman M et al (2013) Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 150(2):384–388
Rohan KJ et al (2016) A protocol for the hamilton rating scale for depression: item scoring rules, rater training, and outcome accuracy with data on its application in a clinical trial. J Affect Disord 200:111–118
Carrozzino D et al (2020) The hamilton rating scales for depression: a critical review of clinimetric properties of different versions. Psychother Psychosom 89(3):133–150
Aas IHM (2011) Guidelines for rating global assessment of functioning (GAF). Ann Gen Psychiatry 10(1):2
Aas IHM, Sonesson O, Torp S (2018) A Qualitative Study of Clinicians Experience with rating of the global assessment of functioning (GAF) Scale. Community Ment Health J 54(1):107–116
Bernd Löwe et al., Gesundheitsfragebogen für Patienten (PHQ D). Komplettversion und Kurzform. Testmappe mit Manual. Fragebögen, Schablonen. 2002, 2. Auflage, Karlsruhe Pfizer.
Aaron T. Beck, Robert-A. Steer, and Gregory K. Brown, Beck Depressions-Inventar : BDI-II ; Revision ; Manual, ed. F.K. M. Hautzinger, Ch. Kühner. 2009 (1996), 2. Auflage, Frankfurt: PSychCorp / Pearsons Assessment.
Schmitt A, Kulzer B, Hermanns N (2015) German version of the GRID Hamilton Rating Scale for Depression (GRID-HAMD), Übersetzung: Forschungsinstitut der Diabetes-Akademie Bad Mergentheim, 2012
Saß H et al (2003) Diagnostisches und Statistisches Manual Psychischer Störzungen - Textrevision - DSM-VI-TR. Hogrefe - Verlag für Psychologie, Göttingen - Bern - Toronto - Seattle
Kroenke K et al (2010) The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry 32(4):345–359
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16(9):606–613
Kuehner C et al (2022) Diagnostic performance and validity of the German Version of the BDI-II - a secondary analysis with data from clinical and nonclinical samples. Psychiatr Prax. https://doi.org/10.1055/a-1753-2298
Saeedi S et al (2021) Neuron-derived extracellular vesicles extracted from plasma show altered size and miRNA cargo as a function of antidepressant drug response. bioRxiv 9:895
Osimo EF et al (2020) Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 87:901–909
Ogyu K et al (2018) Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev 90:16–25
Zorrilla EP et al (2001) The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 15(3):199–226
Guloksuz S et al (2014) The immune system and electroconvulsive therapy for depression. J Ect 30(2):132–137
Yrondi A et al (2018) Electroconvulsive therapy, depression, the immune system and inflammation: a systematic review. Brain Stimul 11(1):29–51
Moschny N et al (2020) Electroconvulsive therapy, changes in immune cell ratios, and their association with seizure quality and clinical outcome in depressed patients. Eur Neuropsychopharmacol 36:18–28
Ahmetspahic D et al (2018) Altered B cell homeostasis in patients with major depressive disorder and normalization of cd5 surface expression on regulatory B cells in treatment responders. J Neuroimmune Pharmacol 13(1):90–99
Miller A (2010) Depression and immunity: a role for T cells? Brain Behav Immun 24:1–8
Kappelmann N et al (2020) Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-sample mendelian randomization study. JAMA Psychiat 78:161
Lamers F et al (2020) Depression profilers and immuno-metabolic dysregulation: Longitudinal results from the NESDA study. Brain Behav Immun 88:174–183
Milaneschi Y et al (2021) Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry 21:1696
de Kluiver H et al (2020) Associations between depressive symptom profiles and immunometabolic characteristics in individuals with depression and their siblings. World J Biol Psychiatry 22:128
Lago SG et al (2021) Exploring cellular markers of metabolic syndrome in peripheral blood mononuclear cells across the neuropsychiatric spectrum. Brain Behav Immun 91:673–682
Qiu W et al (2021) Update on the relationship between depression and neuroendocrine metabolism. Front Neurosci. https://doi.org/10.3389/fnins.2021.728810
Lopizzo N et al (2015) Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front Psychiatry. https://doi.org/10.3389/fpsyt.2015.00068
Faul F et al (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Goodman WM, Spruill SE, Komaroff E (2019) A proposed hybrid effect size plus p-value criterion: empirical evidence supporting its use. Am Stat 73(sup1):168–185
Acknowledgements
The authors would like to thank Karin Hofer, Elisabeth Kappelmann, and Alexandra Kocsis from the MPI study centre along with Evangelos Kokolakis and Julius Ziebula for their assistance with local study organisation. The authors furthermore express their gratitude to Susanne Lucae, Tamara Namendorf, and the MPIP’s BioPRep biobank core unit team for their help with blood sample preparation and storage. The author team recognizes the efforts of all former scientists involved in past ECT research projects at the MPIP. Finally, the authors also express their appreciation to Tanja Brückl for her advice on psychometry and Corina Nagy (McGill University, Canada) and Pablo Lopez (Karolinska Institute, Sweden) for their technical advice on exosome isolation.
Funding
Open Access funding enabled and organized by Projekt DEAL. IvM received funding from Else Kroener-Fresenius-Foundation as well as from the International Max Planck Research School for Translational Psychiatry. JMD received funding from the German Federal Ministry of Education and Research (IMADAPT, FKZ: 01KU1901) and from the Marie Skłodowska-Curie training network PurinesDX.
Author information
Authors and Affiliations
Contributions
IvM and JP jointly developed the DetECT study, drafted the study protocol, and co-wrote the manuscript at hand. NG contributed valuable administrative and scientific support during study development, registration, and conductance, and subedited the manuscript. AE supported the local implementation of the DetECT study in the MPIP’s clinic, provided psychiatric expertise, and reviewed the manuscript. JD provided conceptual scientific advice on purinergic signaling, financial support for purine measurement applications and sensors, and subedited the manuscript. EBB is the study’s principal investigator, oversaw the study conceptualisation and protocol development, provided constant scientific and financial support, and reviewed and edited the manuscript at hand. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
EBB is an editor of the journal European Archives of Psychiatry and Clinical Neuroscience. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be viewed as a conflict of interest.
Ethical approval and consent to participate
The study was approved by the ethics committee of the Ludwig Maximilian University (LMU), in Munich, Germany (approval date: 24/02/2022, Project No. 21–1087). Written informed consent was obtained from all participants before taking part in the study. Protocol modifications (amendments) are reported to the ethics committee of the LMU prior to implementation. Blood and all generated data are stored in the Max Planck Institute’s Biobank (Project No. 338–15), which has also been approved by the same LMU ethics committee.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Mücke-Heim, IA., Pape, J.C., Grandi, N.C. et al. Multiomics and blood-based biomarkers of electroconvulsive therapy in severe and treatment-resistant depression: study protocol of the DetECT study. Eur Arch Psychiatry Clin Neurosci 274, 673–684 (2024). https://doi.org/10.1007/s00406-023-01647-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-023-01647-1