Abstract
Fibromyalgia, a condition characterized by chronic pain, is frequently accompanied by emotional disturbances. Here we aimed to study brain activation and functional connectivity (FC) during processing of emotional stimuli in fibromyalgia. Thirty female patients with fibromyalgia and 31 female healthy controls (HC) were included. Psychometric tests were administered to measure alexithymia, affective state, and severity of depressive and anxiety symptoms. Next, participants performed an emotion processing and regulation task during functional magnetic resonance imaging (fMRI). We performed a 2 × 2 ANCOVA to analyze main effects and interactions of the stimuli valence (positive or negative) and group (fibromyalgia or HC) on brain activation. Generalized psychophysiological interaction analysis was used to assess task-dependent FC of brain regions previously associated with emotion processing and fibromyalgia (i.e., hippocampus, amygdala, anterior insula, and pregenual anterior cingulate cortex [pACC]). The left superior lateral occipital cortex showed more activation in fibromyalgia during emotion processing than in HC, irrespective of valence. Moreover, we found an interaction effect (valence x group) in the FC between the left pACC and the precentral and postcentral cortex, and central operculum, and premotor cortex. These results suggest abnormal brain activation and connectivity underlying emotion processing in fibromyalgia, which could help explain the high prevalence of psychopathological symptoms in this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibromyalgia is a clinical syndrome that mainly affects women [1], characterized by a chronic presence of widespread pain, accompanied by a conglomerate of physical and psychological symptoms. These include fatigue, sleep disturbances, and cognitive dysfunction [2]. Furthermore, depression and anxiety are common comorbidities, with a lifetime prevalence of depression estimated to be higher than 50%, and up to 33% for anxiety [3]. A common finding in fibromyalgia is an imbalance in the presence of positive and negative affect, meaning that patients experience positive affect less frequently than controls, while negative affect is more frequent [4, 5]. This imbalance is not only associated with an increase in the prevalence of depression and anxiety [6], it is also related to higher intensity of pain [7, 8], lower cognitive performance [9], and overall higher severity of fibromyalgia [10]. Because of its relevance for symptomatology, understanding and improving affect in fibromyalgia is an important clinical goal.
Disturbances in emotion processing and regulation have been related to affective instability [11, 12] and might help explain the high comorbidity of fibromyalgia with depression and anxiety. It has been described that patients with fibromyalgia experience emotions with higher intensity and arousal than controls [13, 14]. Additionally, fibromyalgia patients use expressive suppression more frequently as an emotion regulation strategy when compared to controls [14, 15]. Expressive suppression is a strategy frequently associated with psychopathology [16], and it is considered to be maladaptive because when compared to reappraisal, it fails to effectively reduce negative affect and results in increased arousal [13, 14, 17], although findings are not fully consistent [18]. Also, difficulties to identify and describe one's own feelings, known as alexithymia, have been shown to be associated with fibromyalgia and impaired emotion regulation [19,20,21]. Alexithymia has been related to higher impact of the fibromyalgia [22], depression, anxiety, [23, 24] and pain [19, 25], although not all studies report this relationship [26]. In general, emotion processing and regulation disturbances affect emotional state in fibromyalgia patients and are thought to facilitate the development of depression and anxiety.
Recent advances in the understanding of the physiopathology of fibromyalgia can help elucidate the origin of the emotional difficulties that characterize this disorder. One of the current theories describes fibromyalgia as a central sensitization condition [27, 28], which is triggered by neuro-inflammation [29,30,31]. The brain areas that have been associated with neuro-inflammation encompass the precuneus, posterior cingulate cortex, midcingulate cortex, supramarginal gyrus, superior parietal lobe, frontal operculum, dorsolateral prefrontal cortex, precentral and postcentral gyrus, medial prefrontal cortex, and the superior frontal gyrus, including the supplementary motor area [29, 31]. Interestingly, many of these brain areas are related to emotion processing and regulation. Roughly, the amygdala, hippocampus, insula, and midcingulate cortex are associated with the generation of the primary emotional response and salience detection. The ventrolateral prefrontal cortex has been associated with signaling the need for control and calling for additional regulatory resources [32,33,34]. Finally, the dorsolateral prefrontal cortex, supplementary motor area, anterior cingulate cortex, superior parietal lobe, and angular gyrus are related to the implementation of regulation strategies, such as cognitive reappraisal and suppression [32, 35, 36]. Thus, multiple brain regions are orchestrated, to execute a set of cognitive functions that underpin the adequate emotion processing and regulation.
A few previous studies have addressed alterations of brain activity during the processing of emotional stimuli in fibromyalgia [13, 37, 38]. Some of these studies relied on electroencephalography, and one functional magnetic resonance imaging (fMRI) study investigated brain activation associated to the influence of emotions on the processing of painful stimuli [39]. In the current study, we aimed to investigate brain activation and functional connectivity (FC) during emotion processing and regulation in fibromyalgia using fMRI. Specifically, we studied the differences between female fibromyalgia patients and healthy controls (HC) in whole-brain activation and in FC of structures in networks associated with emotional processing and regulation. We focused on female participants because of the higher prevalence of fibromyalgia in this group. Furthermore, we investigated whether individual variation in the clinical presentation of fibromyalgia relates to FC, particularly alexithymia, pain, depression, and anxiety owing to the hypothesized inter-relation between these variables mentioned earlier. To this end, we compared fMRI data acquired from fibromyalgia patients and HC during emotional processing and regulation. We hypothesized higher brain activation in fibromyalgia in the prefrontal cortex, pregenual anterior cingulate cortex (pACC), insula, and amygdala, as well as higher FC between the anterior insula, amygdala and cortical areas involved in emotion processing and regulation, with larger abnormalities in those displaying higher alexithymia and pain, regulated by depression and anxiety.
Materials and methods
Participants
The current analysis is part of a bigger project from the National Institute of Psychiatry in Mexico (internal Research Committee registry number: IC18080.0) which aims to study clinical and neuroimaging correlates of emotion processing and regulation in fibromyalgia. For the present analysis, we included participants who performed an emotion processing and regulation task (EPRT) during fMRI: 34 fibromyalgia patients and 33 HC (all females). After fMRI quality control (see fMRI preprocessing in the Online Resource document), data from 30 patients with fibromyalgia (mean age = 41.8 years; years of education = 15.5) and 31 HC (mean age = 41.2 years; years of education = 16.8) were analyzed. For details on recruitment and selection criteria, see Online Resource. All participants were right-handed and the groups were matched with respect to age and years of education. All participants provided written informed consent. The protocol was approved by Research Ethics Committee of the National Institute of Psychiatry “Ramon de la Fuente Muñiz” in Mexico City.
Clinical measures
A series of scales and interviews were administered to assure the compliance with inclusion and exclusion criteria, and to characterize the sample. To confirm right laterality, the Edinburgh Handedness Inventory Short Form was administered [40]. All participants with fibromyalgia had previously received the diagnosis by a rheumatologist or an internist. To confirm (or exclude for HC) the diagnosis of fibromyalgia, we used the American College of Rheumatology 2016 criteria [41]. To exclude major psychiatric disorders in the sample or any disorder in HC, as well as to document other psychiatric comorbidities in the fibromyalgia group (such as depression and anxiety), the Mini International Neuropsychiatric Interview-Plus [42] was administered by a trained psychiatrist. To measure severity of depressive and anxiety, we applied the 17-items Hamilton Depression Rating Scale (HAMD) [43, 44] and the Hamilton Anxiety Rating Scale (HAMA) [45]. Other clinical variables were measured using self-rating scales: the tendency to employ cognitive reappraisal or suppression with the Emotional Regulation Questionnaire [12], alexithymia with the Toronto Alexithymia Scale [46, 47], regular affective state in the past week with the Positive and Negative Affect Schedule [48, 49]. Participants filled the Socio-economic levels Questionnaire of the Mexican Association of Market Research and Public Opinion Agencies Questionnaire (AMAI NSE 8 × 7), which is a standardized instrument to measure socio-economic level in Mexican households, and filled a form with sociodemographic data and clinical characteristics of fibromyalgia (i.e., duration and treatment). Finally, the Fibromyalgia Impact Questionnaire [50] and the McGill Pain Questionnaire [51, 52] were administrated to participants in the fibromyalgia group to evaluate the severity of fibromyalgia and the characteristics of pain, respectively.
Procedure and fMRI task
Evaluations for each participant were conducted over two sessions with a median time interval of 8 days (range 0–20). During the first session, the interviews and scales mentioned were applied. On the second session, participants were trained to perform the EPRT, and next they underwent the MRI session. Depression, anxiety and affect measurements were updated during the second interview when time interval between sessions was longer than eight days.
For the EPRT, three regulation instructions were implemented: Attend, Reappraise, and Suppress. During the Attend conditions, participants were asked to observe the pictures and allow themselves to experience any emotional response elicited by the stimuli without trying to manipulate the emotional experience. The Reappraise condition had two variants depending on the valence of the stimuli: Increase for positive pictures and Decrease for negative ones. In the case of Increase, participants were asked to boost the positive emotional experience elicited by the stimuli by reinterpreting the presented picture in a more positive self-related manner. In the case of Decrease, participants were asked to reduce the negative emotion elicited by the stimuli by interpreting it as something distant and not dangerous for them or by changing the meaning of the situation to something less negative. For the Suppress conditions (positive and negative), participants were asked to avoid any emotional expression elicited by the stimuli, so that someone watching them would not be able to infer their emotional state. In total, there were seven conditions: Attend neutral, Attend negative, Attend positive, Reappraise negative, Reappraise positive, Suppress negative, and Suppress positive. The task was implemented in a block design (Fig. 1). After each block, participants rated on a visual analog scale the intensity of the emotion elicited, arousal and physical pain. See Online Resource for details on the task.
fMRI data acquisition
Whole-brain functional and anatomical images were acquired using a 3.0T Philips Ingenia Magnetic Resonance Imaging scanner with a 32-channel phased array head coil. See Online Resource for details on the sequences.
Clinical and behavioral analyses
Demographic and clinical characterization of the sample was evaluated using non-parametric (Wilcoxon test) and parametric tests. Permutation tests with 1000 iterations were executed for analyzing the behavioral data from the EPRT. Behavioral analyses were done first comparing the ratings (emotional intensity, arousal, and pain intensity) for the combination of all instructions (Regulate, Suppress, and Attend) and valences (neutral, positive, and negative), leading to compare the seven conditions. In consideration of the lack of intra-group effects of conditions on ratings and brain activation (see “Behavioral” and “Whole-brain activation” “Results” sections), we analyzed behavioral data across instructions, leading effectively to comparing the effect of valence (neutral, positive, and negative). To assess the differences between groups in emotion intensity, arousal, and pain, we performed a non-parametric contrast between groups for each variable per task condition (and valence across instructions). Because depression and anxiety may relate to intensity of the emotion, arousal, and pain, we performed Spearman’s correlation between ratings during each valence and the scores of HAMD and HAMA for the fibromyalgia group. Statistics were performed using R 3.6.2 (R Core Team, 2019).
fMRI preprocessing
The quality of the acquired image sequences was assessed using MRIQC Toolbox [53]. Preprocessing and first-level analysis were executed with SPM12 (Statistical Parametric Mapping, Wellcome Institute for Cognitive Neurology, London, UK) [54]. For details, see the Online Resource.
For the first-level analysis, data was analyzed in the context of the general linear model, with the time series of each participant convolved with the canonical hemodynamic response function and a 128 s high-pass filter applied. The model included the seven conditions (onsets of each picture and durations) plus the instructions, the visual analog scales and, the temporal and dispersion derivatives as regressors. Brain activation during fixation was modeled as implicit baseline.
Whole-brain activation analysis
Group analyses were performed at the whole-brain level. We planned two repeated measures ANCOVAs: one with the negative conditions (Attend negative, Reappraise negative, and Suppress negative) and one with the positive conditions (Attend positive, Reappraise positive, and Suppress positive) as the within-subject factor. Group (fibromyalgia and HC) was set as the between-subjects factor, and age and education were entered as covariates. Because exploration of the data across all participants revealed lack of significant activations for the main effect of conditions (across groups, and within HC and fibromyalgia separately; even at uncorrected threshold, which was in line with the behavioral results; see “Clinical and behavioral analyses” and “Results”), we set up a 2 × 2 ANCOVA model with valence (positive and negative, across instructions) as the within-subject factor, groups (fibromyalgia and HC) as the between-subjects factor, and age and education as covariates. In this model, the first-level contrasts used were all negative > implicit baseline, and all positive > implicit baseline. This decision allowed us to assess emotion processing and regulation according to the valence of the stimuli, but without differentiating processing from regulation. We modeled the main effect of group, and valence × group interaction. To explore relations of depression and anxiety, the peak activation of the significant clusters was extracted and correlated with the HAMD and HAMA scores.
Functional connectivity: psychophysiological interaction analysis
The FC was estimated using a generalized Psychophysiological interaction (gPPI) approach. We chose the following seeds (left and right) based on their affiliation with the amygdala–hippocampal network and salience network: hippocampus, amygdala, and anterior insula from the Hammers atlas [55, 56], and pACC five mm spheres were created based on literature about anterior cingulate cortex coordinates relevant for emotion regulation (MNI ± 6 36 10) [36, 57]. In total, eight seeds were defined.
To perform the gPPI analyses, the time series for each seed was extracted and deconvolved to uncover neural activity. Next, the interaction term was created by multiplying the deconvolved seed time series and the task design time series. This interaction term was convolved with the hemodynamic response function to form the gPPI regressor, and the resulting time series was regressed against the rest of the brain to obtain the valence specific FC estimates. Thus, for each seed, a general linear model was estimated in the first-level analysis. In these models, the psychological terms for the gPPI consisted of the contrast between each task condition (i.e., Attend positive) and the implicit baseline, similar to the whole-brain activation analysis model. The other regressors were the time series of the seed and the interaction term. Additionally, temporal and dispersion derivatives were included. Following the across-instruction approach of the main brain activity analyses, the contrasts used in the second-level analysis were: all negative > implicit baseline, and all positive > implicit baseline. To test for the main effects of group and the valence x group effect, we performed an ANCOVA 2 × 2 (valence x group). Next, to test whether depression and anxiety severity moderated the relation between alexithymia, pain, and FC, we extracted the mean connectivity term from the clusters with significant correlation with the clinical variables and performed a partial correlation analysis to control for the contribution of depression and anxiety. The SPM 12 gPPI toolbox was used to perform the gPPI analyses and R for the partial correlations.
Statistical significance
For all analyses (clinical, behavioral, and fMRI), significant level was set to p < 0.05 two-tailed, and False discovery rate (FDR) corrected for multiple comparisons, at a cluster-level for the fMRI data. For the gPPI analysis, an additional Bonferroni correction was applied to account for the number of seeds that were explored.
Results
Demographic and clinical characteristics
The demographic characteristics of the 30 fibromyalgia and 31 HC participants are summarized in Table 1. Among the fibromyalgia participants, 56.67% used medication daily (pregabalin being the most prescribed) and 63% fulfilled criteria for at least one current psychiatric disorder, being major depressive disorder the most common (46.7%). For clinical and psychiatric details specific to the fibromyalgia group, see Tables 2S and 3S in the Online Resource. As shown in Table 2, in comparison to HC, fibromyalgia participants presented more pain (W = 925.5, p < 0.001), depressive and anxious symptoms (W = 914.0, p < 0.001, and W = 926.0, p < 0.001, respectively), alexithymia (W = 720.0 p < 0.001), negative affect (W = 774.0, p < 0.001), and less positive affect (W = 190.0, p < 0.001). The use of cognitive reappraisal in daily life did not differ between groups (t(59) = − 0.4, p = 0.73); for suppression, a non-significant tendency for higher use was found in fibromyalgia (W = 590.0, p = 0.073).
Behavioral
We only observed an effect of valence (neutral, positive, and negative), and not of condition (e.g., Reappraise positive, as summarized in Fig. 2 and 1S. Based on this result, we decided to continue the analyses with valence as the independent variable. For effects on pain in fibromyalgia, pain intensity was higher during negative valence compared to neutral (Z = 2.31 p = 0.03) and positive (Z = 3.44 p = 0.002) as shown in Fig. 2 panel C; depression (and possibly anxiety) might explain pain during negative valence as these two variables were positively correlated (r(26) = 0.43, p = 0.02, and r(26) = 0.36, p = 0.06, respectively). Pain did not differ between valence for HC (mean = 0.8 points, X2(2) = 1.0, p = 0.6).
Behavioral results of the emotion regulation task. A Intensity of emotion per condition: the visual analog scale (VAS) points represent the valence and intensity of the emotion experienced with 0 corresponds to the most intense negative emotion, 5 is a neutral state and 10 is the most intense positive emotion. B Arousal per condition: The visual analog scale (VAS) points represent the arousal with 0 being the minimum level and 10 is the most intense. C Pain per condition: the visual analog scale (VAS) points represent the pain intensity with 0 being no pain and 10 is the most intense pain. Mean and SD in the boxes. * Intergroup significant differences (all differences were significant at p < 0.001)
For emotional intensity, fibromyalgia patients experienced emotions more negatively than HC, both for negative (Z = 8.0, p < 0.001), and positive stimuli (Z = 3.0, p = 0.009). The arousal was also higher in fibromyalgia for negative stimuli (Z = − 4.0 p < 0.001). Depression and anxiety were not correlated to emotional intensity nor arousal in fibromyalgia (Online Resource Table 4S).
Whole-brain activation
For the 2 × 2 ANCOVA (valence x group) model, we found a significant cluster for the group main effect in the left superior lateral occipital cortex (MNI coordinates: -30 -79 23; F = 26.53, p = 0.028). The post hoc t-test for the contrast fibromyalgia > HC was significant for that cluster (Fig. 3; t(116) = 5.15, p = 0.023, k = 77). The BOLD activation of this cluster correlated positively with depression (r(25) = 0.58, p = 0.001), and anxiety (r(25) = 0.66, p < 0.001) in fibromyalgia.
Functional connectivity: psychophysiological interactions
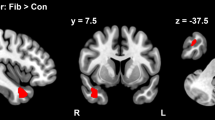
We explored the task-modulated FC of hippocampus, amygdala, anterior insula, and pACC during processing and regulation of emotions. In our analysis, only the left pACC seed showed a valence x group interaction (Table 3). The right post- and precentral cortex, and the left central operculum, premotor and postcentral cortex showed higher FC with the left pACC in fibromyalgia participants than HC during the processing of positive valence stimuli, while during the processing of negative valence stimuli, the FC was lower in these regions (Fig. 4). In fibromyalgia, the FC of left pACC with significant clusters did not correlate with depression nor anxiety (Table 4). The rest of the seeds (bilateral hippocampus, amygdala, anterior insula, and right pACC) did not show significant effects of group nor a valence × group interaction.
Left ACC task-modulated functional connectivity during emotion regulation. Three clusters were significative for the valence x group interaction effect: A right precentral/postcentral cortex, B right frontal operculum, and C left central operculum, posterior insula, premotor and postcentral cortex. ACC, anterior cingulate cortex; FM, fibromyalgia group; HC, control group; +, regulation of positive valence stimuli; −, regulation of negative valence stimuli
Finally, a correlation analysis was performed between the FC of each seed and the clinical variables alexithymia and pain in the fibromyalgia group. The Difficulty to identify feelings (DIF) subscale was the only alexithymia measure that correlated to the FC between right anterior insula and a cluster encompassing parts of the right superior frontal gyrus, the supplementary motor area and the dorsal anterior cingulate cortex (MNI coordinates: x = 18, y = 5, z = 47, size: 43 voxels, r(28) = 0.61, p = 0.01). Adding depression in a partial correlation moderated the relation between right insula seeded FC and DIF, so it was no longer significant (r(27) = 0.49, p = 0.23). When the correlation analysis was repeated including the control group, no correlation was found. The FC of other seeds did not correlate with alexithymia nor pain.
Discussion
In this study, we investigated brain activation and FC during emotion processing and regulation in fibromyalgia, using fMRI. We found higher activation of lateral occipital cortex in fibromyalgia during processing of emotional pictures, irrespective of valence. We also found altered valence-dependent FC of the left pACC with the opercular, premotor, post- and precentral cortex during the processing and regulation of emotional stimuli in fibromyalgia. More specifically, we observed higher pACC seeded FC during positive emotion processing and lower FC during negative emotion processing. Our behavioral analysis showed more negative emotions in fibromyalgia than in HC. Taken together, these results suggest that higher negative mood and pain in fibromyalgia are associated with abnormal processing of negative and positive emotional stimuli, which might underpin the emotional difficulties present in fibromyalgia and its effect on pain.
The lateral occipital cortex was found to be hyper-activated in fibromyalgia compared to HC, irrespective of valence, but with variations according to the severity of depression. The lateral occipital cortex has been related to visual attention [58], object perception [59, 60], but also to pain modulation [61]. A recent study found that the decrease in resting-state FC between the occipital cortex and the pACC following transcranial magnetic stimulation relates to a reduction in the affective dimension of pain in fibromyalgia [62], suggesting the occipital cortex has functional relevance for the affective processing of pain. Hypo-activation of visual areas has been already described in other populations with high levels of alexithymia during subliminal emotional stimuli [63]. Here, the observed higher activation of the occipital gyrus during overt emotional stimuli may suggest higher use of visual cues to process and regulate emotions (in opposition to cognitive processes). Additional to pain modulation and alexithymia, emotion processing or depression could explain the differences in occipital activation since this region was found affected in a previous study using a similar emotion processing and regulation task in patients with remitted depression [64], and from our results in the correlation with severity of depression.
Besides the lateral occipital cortex, no other regions showed activation differences, including those from the hypothesis (insula, amygdala, prefrontal and pACC). This could be due to a compensation mediated by changes in the FC as found for the pACC. For the other hypothesized regions, amygdala and prefrontal cortex could be less involved with the emotion processing and regulation difficulties, while the insula alterations seem to depend more on psychological characteristics of the fibromyalgia group, as found in the correlation of FC and clinical variables.
In regard to the pACC, it is an integrative hub associated with the convergence of salient interoceptive, sensitive and emotional stimuli for further processing and coordination of potential motor responses [65]. Furthermore, it is an area rich in opioid receptors [66], thereby participating in the affective assessment of pain [67]. Finally, pACC activation, connectivity, and structure have been implicated in the top-down regulation of negative affect and pain [69,70,70]. Previously, pACC FC with other structures from a pain inhibitory network was found to be reduced in fibromyalgia [71], and its activation during pain has been related to the availability of μ-opioid receptors and to the affective intensity of pain [72].
We found a hyper-connectivity of pACC during positive stimuli processing and a reduced connectivity during negative stimuli processing with the precentral gyrus, postcentral gyrus, central operculum, and superior frontal gyrus. The precentral and postcentral gyri have been found important for the discrimination of emotions and the representation of valence [73, 74], and to be activated during reappraisal and suppression [32, 75]. It has been reported that these areas are hypoactive in women with premenstrual dysphoric disorder [76], a condition characterized also by emotional dysregulation and pain. The central operculum has been implicated in emotion and pain processing in pathological conditions. For example, in functional dystonia and alcohol dependence, pACC was found hypoactive while performing emotion regulation tasks [77, 78], and in masochist patients, its FC was impaired during a task that involved emotional and painful stimuli [79]. The valence-related alteration of the pACC FC to areas related to interoception and emotion processing and regulation that we found might help explain the relationship between negative affect and higher pain intensity [7, 80], and the deficient pain modulation by positive emotional stimuli [39] in fibromyalgia.
In consideration of the mentioned evidence from the literature and our results, where negative conditions were characterized by higher pain intensity in fibromyalgia and more negative emotional experience (between-groups comparison), we interpret that in fibromyalgia, the processing of emotional stimuli is disrupted according to its valence, given by the affected FC between areas involved in the processing of valence and internal representations encoding. In other words, the pACC FC is affected in fibromyalgia during the processing of emotional stimuli and how it is affected depends on whether the valence of the stimuli is positive or negative.
The involvement of the pACC in linking negative affect and pain is not exclusive from fibromyalgia and might be related to neuro-inflammation. A metabolic marker of neuro-inflammation in the pACC was related to negative affect in patients with chronic low back pain [81]. Although the studies on neuro-inflammation in fibromyalgia [29, 31, 82] have not shown neuro-inflammation this region, neuro-inflammation has been found in areas connected to the pACC such as the midcingulate cortex [83]. Thus, one possibility is that neuro-inflammation affects the pACC through its connection to areas with neuro-inflammation. Alternatively, neuro-inflammation is present in the pACC in fibromyalgia, but it has not been observed due to the employed methodology. Whether neuro-inflammation affects the pACC in fibromyalgia requires further research. In case it is proved, neuro-inflammation could become a treatment target to improve affect and pain modulation in patients with fibromyalgia.
Given the involvement of the anterior insula in emotion processing and regulation [75] and in fibromyalgia symptomatology [84], we studied its FC. We found that in fibromyalgia, during the regulation of negative stimuli, the anterior insula FC to the superior frontal gyrus, supplementary motor area, and dorsal anterior cingulate cortex, areas that are important for emotion regulation, was positively correlated with the DIF, a factor of the alexithymia construct. This finding is in accordance to previously described negative correlation of anterior insula activation with alexithymia [85]. Notably, the DIF relation to anterior insula connectivity was explained by depression according to our partial correlation analysis results.
Limitations and future directions
Some limitations need to be taken into account. As for most of the studies in fibromyalgia, our results are limited to women. This is a limitation insofar that we do not know if the findings extend to men. Nevertheless, as a first study, we limited the sample to women to enhance homogeneity, taking into account that the prevalence is considerably larger in women (3.98%, for women vs. 0.01% for men) [1]. Furthermore, participants over 50 years old were excluded to ease task-training, in spite of representativity of elderly patients where the prevalence of fibromyalgia is high [86]. Another limitation pertaining our sample is the presence of physical and mental health comorbidities in the fibromyalgia group with some patients being medicated with drugs that affect central nervous system such as antidepressants. Nevertheless, we required participants to have a stable dose of their medication and to avoid rescue doses 24 h before the brain scan. Although the medication and the mental health disorders could affect the task performance and brain activity, mental comorbidities are highly prevalent among patients with fibromyalgia, which make our findings more generalizable [3, 87], exempting for those using opioids since they were excluded from the study. Furthermore, we performed analyses to control for depression and anxiety.
An important limitation is the lack of effects of condition in the behavioral and the ANCOVA 3 × 2 analyses (condition [reappraise, suppress, attend] x group) in the fMRI analysis. This task has been successfully applied without the suppress condition in a Dutch sample of people with remitted depression [64]. One possible explanation for the lack of clear differences could be that it may have been difficult for our sample to follow the three different regulation instructions (even after training them), as it is suggested by a study using a similar task in a Mexican sample where differences in the effect size of cognitive reappraisal were found according to the procedures to instructing participants [88]. Another possibility is that it may be more difficult for people with fibromyalgia to adequately perform the different emotion regulation strategies than for people with remitted depression. Furthermore, our fMRI results must be taken cautiously, especially the whole-brain activation analysis, since the multiple comparison correction approach was relatively liberal (p < 0.05 FDR). In regard to the FC analysis, an additional correction to account for the number of seeds was applied. Finally, our interpretations concerning pain intensity were conceived based on the behavioral results of the task. However, to assess how emotion regulation affects the neural underpinnings of pain, tasks that include painful stimuli along with emotional ones should be applied.
Conclusions
Taken together, our findings suggest that difficulties in emotion processing and regulation in fibromyalgia are related to alterations in activation of lateral occipital cortex, and in FC of the pACC, a central hub for emotion and pain processing, to other important emotion processing and regulation areas. These alterations might imply that a stronger involvement of regions associated with pain modulation is needed to process emotional stimuli (including valence and pain) in fibromyalgia. As standardized treatment and pharmacotherapy in fibromyalgia are often unsuccessful [89], psychotherapeutic strategies are being used more frequently to treat pain as well as the emotional difficulties and psychiatric comorbidities. Some of these therapies emphasize emotional awareness and regulation [15, 90, 91]. Other therapeutic strategies include neuromodulation techniques [92, 93]. Longitudinal studies are needed to better understand the neural mechanisms of improvement with those therapies. The findings of the current study highlight some of the brain structures for which activation or connectivity might change after treatment.
Data availability
The dataset is described in Scientific Data [94] and publicly available.
References
Heidari F, Afshari M, Moosazadeh M (2017) Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017:1–13
Clauw DJ (2009) Fibromyalgia: an overview. Am J Med 122:S3–S13
Kleykamp BA, Ferguson MC, McNicol E, Bixho I, Arnold LM, Edwards RR, Fillingim R, Grol-Prokopczyk H, Turk DC, Dworkin RH (2021) The prevalence of psychiatric and chronic pain comorbidities in fibromyalgia: an acttion systematic review. Semin Arthritis Rheum 51:166–174
Finan PH, Zautra AJ, Davis MC (2009) Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med 2009:71
Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, Davis MC (2005) Fibromyalgia: evidence for deficits in positive affect regulation. Psychosom Med 67:147–155
Hassett AL, Simonelli LE, Radvanski DC, Buyske S, Savage SV, Sigal LH (2008) The relationship between affect balance style and clinical outcomes in fibromyalgia. Arthritis Care Res 59:833–840
Kratz AL, Davis MC, Zautra AJ (2007) Pain acceptance moderates the relation between pain and negative affect in female osteoarthritis and fibromyalgia patients. Ann Behav Med 33:291–301
Staud R (2004) Predictors of clinical pain intensity in patients with fibromyalgia syndrome. Curr Rheumatol Rep 6:281–286
Galvez-Sánchez CM, Reyes del Paso GA, Duschek S (2018) Cognitive impairments in fibromyalgia syndrome: associations with positive and negative affect, alexithymia, pain catastrophizing and self-esteem. Front Psychol 2018:9
Estévez-López F, Gray CM, Segura-Jiménez V, Soriano-Maldonado A, Álvarez-Gallardo IC, Arrayás-Grajera MJ, Carbonell-Baeza A, Aparicio VA, Delgado-Fernández M, Pulido-Martos M (2015) Independent and combined association of overall physical fitness and subjective well-being with fibromyalgia severity: the al-ándalus project. Qual Life Res 24:1865–1873
Nezlek JB, Kuppens P (2008) Regulating positive and negative emotions in daily life. J Pers 76:561–580
Gross JJ, John OP (2003) Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 85:348–362
Rossello F, Munoz MA, Duschek S, Montoya P (2015) Affective modulation of brain and autonomic responses in patients with fibromyalgia. Psychosom Med 77:721–732
van Middendorp H, Lumley MA, Jacobs JW, van Doornen LJ, Bijlsma JW, Geenen R (2008) Emotions and emotional approach and avoidance strategies in fibromyalgia. J Psychosom Res 64:159–167
Bowers HM, Pincus T, Jones G, Wroe AL (2019) Investigating the role of beliefs about emotions, emotional suppression and distress within a pain management programme for fibromyalgia. Br J Pain 13:112–120
McRae K, Gross JJ (2020) Emotion regulation. Emotion (Washington, DC) 20:1–9
Rhudy JL et al (2013) Emotional modulation of pain and spinal nociception in fibromyalgia. Pain 154(7):1045–1056
Rhudy JL, DelVentura JL, Terry EL, Bartley EJ, Olech E, Palit S, Kerr KL (2013) Emotional modulation of pain and spinal nociception in fibromyalgia. Pain 154:1045–1056
Di Tella M, Ghiggia A, Tesio V, Romeo A, Colonna F, Fusaro E, Torta R, Castelli L (2017) Pain experience in fibromyalgia syndrome: the role of alexithymia and psychological distress. J Affect Disord 208:87–93
Marchi L, Marzetti F, Orrù G, Lemmetti S, Miccoli M, Ciacchini R, Hitchcott PK, Bazzicchi L, Gemignani A, Conversano C (2019) Alexithymia and psychological distress in patients with fibromyalgia and rheumatic disease. Front Psychol 2019:10
Taylor GJ (2000) Recent developments in alexithymia theory and research. Can J Psychiatry 45:134–142
Geenen R, van Ooijen-van der Linden L, Lumley MA, Bijlsma JWJ, van Middendorp H (2012) The match–mismatch model of emotion processing styles and emotion regulation strategies in fibromyalgia. J Psychosom Res 72:45–50
Ghiggia A, Romeo A, Tesio V, Tella MD, Colonna F, Geminiani GC, Fusaro E, Castelli L (2017) Alexithymia and depression in patients with fibromyalgia: when the whole is greater than the sum of its parts. Psychiatry Res 255:195–197
Martínez MP, Sánchez AI, Miró E, Lami MJ, Prados G, Morales A (2015) Relationships between physical symptoms, emotional distress, and pain appraisal in fibromyalgia: the moderator effect of alexithymia. J Psychol 149:115–140
Huber A, Suman AL, Biasi G, Carli G (2009) Alexithymia in fibromyalgia syndrome: associations with ongoing pain, experimental pain sensitivity and illness behavior. J Psychosom Res 66:425–433
Montoro CI, Reyes del Paso GA, Duschek S (2016) Alexithymia in fibromyalgia syndrome. Personality Individ Differ 102:170–179
Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL (2003) Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 48:1420–1429
Clauw DJ, Tzadok R (2021) The neuroscience of fibromyalgia and central sensitization. In: Ablin JN, Shoenfeld Y (eds) Fibromyalgia syndrome. Springer International Publishing, Cham, pp 183–192
Albrecht DS, Forsberg A, Sandström A, Bergan C, Kadetoff D, Protsenko E, Lampa J, Lee YC, Höglund CO, Catana C, Cervenka S, Akeju O, Lekander M, Cohen G, Halldin C, Taylor N, Kim M, Hooker JM, Edwards RR, Napadow V, Kosek E, Loggia ML (2019) Brain glial activation in fibromyalgia—a multi-site positron emission tomography investigation. Brain Behav Immun 75:72–83
Ryabkova VA, Churilov LP, Shoenfeld Y (2019) Neuroimmunology: What role for autoimmunity, neuroinflammation, and small fiber neuropathy in fibromyalgia, chronic fatigue syndrome, and adverse events after human papillomavirus vaccination? Int J Mol Sci 2019:20
Seo S, Jung Y-H, Lee D, Lee WJ, Jang JH, Lee J-Y, Choi S-H, Moon JY, Lee JS, Cheon GJ, Kang D-H (2021) Abnormal neuroinflammation in fibromyalgia and crps using [11c]-(r)-pk11195 pet. PLoS ONE 16:e0246152
Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014) Neural network of cognitive emotion regulation—an ale meta-analysis and macm analysis. Neuroimage 87:345–355
Levy BJ, Wagner AD (2011) Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 1224:40–62
Zhang L, Ai H, Opmeer EM, Marsman J-BC, van der Meer L, Ruhé HG, Aleman A, van Tol M-J (2020) Distinct temporal brain dynamics in bipolar disorder and schizophrenia during emotion regulation. Psychol Med 50:413–421
Ochsner KN, Gross JJ (2008) Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci 17:153–158
Morawetz C, Bode S, Baudewig J, Heekeren HR (2017) Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc Cogn Affect Neurosci 12:569–585
Villafaina S, Sitges C, Collado-Mateo D, Fuentes-García JP, Gusi N (2019) Influence of depressive feelings in the brain processing of women with fibromyalgia: an eeg study. Med (Baltim) 98:e15564
Fischer-Jbali LR, Montoro CI, Montoya P, Halder W, Duschek S (2021) Central nervous activity during implicit processing of emotional face expressions in fibromyalgia syndrome. Brain Res 1758:147333
Kamping S, Bomba IC, Kanske P, Diesch E, Flor H (2013) Deficient modulation of pain by a positive emotional context in fibromyalgia patients. Pain 154:1846–1855
Veale JF (2014) Edinburgh handedness inventory—short form: a revised version based on confirmatory factor analysis. Laterality: asymmetries of Body. Brain Cogn 19:164–177
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B (2016) 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 46:319–329
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(20):22–33
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychology 6:278–296
Ramos-Brieva JA, Cordero-Villafafila A (1988) A new validation of the hamilton rating scale for depression. J Psychiatr Res 22:21–28
Lobo A, Chamorro L, Luque A, Dal-Ré R, Badia X, Baró E, de Validaciónen Español de Escalas GP (2002) Validation of the Spanish versions of the Montgomery-Asberg depression and hamilton anxiety rating scales. Med Clin (Barc) 118:493–499
Bagby RM, Parker JDA, Taylor GJ (1994) The twenty-item toronto alexithymia scale—I. Item selection and cross-validation of the factor structure. J Psychosom Res 38:23–32
Moral De la Rubia J (2008) Psychometric properties in Mexico of the Toronto Alexithymia Scale with 20 items. Revista Electrónica de Psicología Iztacala 11:97–114
Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070
Moral De la Rubia J (2011) The positive and negative affect scale (PANAS) in Mexican married couples. Ciencia Ergo Sum 18:117–125
Burckhardt CS, Clark SR, Bennett RM (1991) The fibromyalgia impact questionnaire: development and validation. J Rheumatol 18:728–733
Melzack R (1975) The mcgill pain questionnaire: major properties and scoring methods. Pain 1:277–299
Lázaro C, Bosch F, Torrubia R, Banos JE (1994) The development of a Spanish questionnaire for assessing pain: preliminary data concerning reliability and validity. Eur J Psychol Assess 10:145–151
Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ (2017) Mriqc: advancing the automatic prediction of image quality in mri from unseen sites. PLoS ONE 12:e0184661–e0184661
Friston K (2007) Chapter 2—statistical parametric mapping. In: Friston K, Ashburner J, Kiebel S, Nichols T, Penny W (eds) Statistical parametric mapping. Academic Press, London, pp 10–31
Faillenot I, Heckemann RA, Frot M, Hammers A (2017) Macroanatomy and 3d probabilistic atlas of the human insula. Neuroimage 150:88–98
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS (2003) Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 19:224–247
Schlochtermeier LH, Kuchinke L, Pehrs C, Urton K, Kappelhoff H, Jacobs AM (2013) Emotional picture and word processing: an FMRI study on effects of stimulus complexity. PLoS ONE 8:e55619
Oliva V, Gregory R, Brooks JCW, Pickering AE (2022) Central pain modulatory mechanisms of attentional analgesia are preserved in fibromyalgia. Pain 163:125–136
Maratos FA, Anderson SJ, Hillebrand A, Singh KD, Barnes GR (2007) The spatial distribution and temporal dynamics of brain regions activated during the perception of object and non-object patterns. Neuroimage 34:371–383
Grill-Spector K, Kourtzi Z, Kanwisher N (2001) The lateral occipital complex and its role in object recognition. Vision Res 41:1409–1422
Reis GM, Dias QM, Silveira JWS, Del Vecchio F, Garcia-Cairasco N, Prado WA (2010) Antinociceptive effect of stimulating the occipital or retrosplenial cortex in rats. J Pain 11:1015–1026
Argaman Y, Granovsky Y, Sprecher E, Sinai A, Yarnitsky D, Weissman-Fogel I (2021) Clinical effects of repetitive transcranial magnetic stimulation of the motor cortex are associated with changes in resting-state functional connectivity in patients with fibromyalgia syndrome. J Pain 23:595–615
Donges U-S, Suslow T (2017) Alexithymia and automatic processing of emotional stimuli: A systematic review. Rev Neurosci 28:247–264
van-Kleef RS, Marsman J-BC, van-Valen E, Bockting CLH, Aleman A, van Tol M-J (2022) Neural basis of positive and negative emotion regulation in remitted depression. NeuroImage Clin 34:102988
Palomero-Gallagher N, Hoffstaedter F, Mohlberg H, Eickhoff SB, Amunts K, Zilles K (2018) Human pregenual anterior cingulate cortex: structural, functional, and connectional heterogeneity. Cereb Cortex 29:2552–2574
Jones AKP, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, Cunningham VJ, Itoh M, Fukuda H, Jones T (1991) In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci Lett 126:25–28
Girard-Tremblay L, Auclair V, Daigle K, Léonard G, Whittingstall K, Goffaux P (2014) Sex differences in the neural representation of pain unpleasantness. J Pain 15:867–877
Niedtfeld I, Kirsch P, Schulze L, Herpertz SC, Bohus M, Schmahl C (2012) Functional connectivity of pain-mediated affect regulation in borderline personality disorder. PLoS ONE 7:e33293
Phillips ML, Ladouceur CD, Drevets WC (2008) A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13:833–857
Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wüst S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A (2011) City living and urban upbringing affect neural social stress processing in humans. Nature 474:498–501
Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J (2012) Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain 8:32
Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta J-K, Clauw DJ, Harris RE (2016) Endogenous opioidergic dysregulation of pain in fibromyalgia: a pet and fmri study. Pain 157:2217–2225
Cao L, Xu J, Yang X, Li X, Liu B (2018) Abstract representations of emotions perceived from the face, body, and whole-person expressions in the left postcentral gyrus. Front Hum Neurosci 12:256
Baucom LB, Wedell DH, Wang J, Blitzer DN, Shinkareva SV (2012) Decoding the neural representation of affective states. Neuroimage 59:718–727
Li W, Yang P, Ngetich RK, Zhang J, Jin Z, Li L (2021) Differential involvement of frontoparietal network and insula cortex in emotion regulation. Neuropsychologia 161:107991
Petersen N, Ghahremani DG, Rapkin AJ, Berman SM, Liang L, London ED (2018) Brain activation during emotion regulation in women with premenstrual dysphoric disorder. Psychol Med 48:1795–1802
Espay AJ, Maloney T, Vannest J, Norris MM, Eliassen JC, Neefus E, Allendorfer JB, Chen R, Szaflarski JP (2018) Dysfunction in emotion processing underlies functional (psychogenic) dystonia. Mov Disord 33:136–145
Jansen JM, van den Heuvel OA, van der Werf YD, de Wit SJ, Veltman DJ, van den Brink W, Goudriaan AE (2019) Emotion processing, reappraisal, and craving in alcohol dependence: a functional magnetic resonance imaging study. Front Psychiatry 2019:10
Kamping S, Andoh J, Bomba IC, Diers M, Diesch E, Flor H (2016) Contextual modulation of pain in masochists: involvement of the parietal operculum and insula. Pain 157:445
Galvez-Sánchez CM, Montoro CI, Duschek S, del Paso GAR (2020) Pain catastrophizing mediates the negative influence of pain and trait-anxiety on health-related quality of life in fibromyalgia. Qual Life Res 29:1871–1881
Albrecht DS, Kim M, Akeju O, Torrado-Carvajal A, Edwards RR, Zhang Y, Bergan C, Protsenko E, Kucyi A, Wasan AD, Hooker JM, Napadow V, Loggia ML (2021) The neuroinflammatory component of negative affect in patients with chronic pain. Mol Psychiatry 26:864–874
Jung C, Ichesco E, Ratai E-M, Gonzalez RG, Burdo T, Loggia ML, Harris RE, Napadow V (2020) Magnetic resonance imaging of neuroinflammation in chronic pain: a role for astrogliosis? Pain 161:1555–1564
Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Lozano AM, Mayberg HS (2008) Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex 18:1374–1383
Liu H-Y, Chou K-H, Lee P-L, Wang Y-F, Chen S-P, Lai K-L, Lin C-P, Wang S-J, Chen W-T (2021) Right anterior insula is associated with pain generalization in patients with fibromyalgia. Pain 163:4
Kano M, Fukudo S (2013) The alexithymic brain: the neural pathways linking alexithymia to physical disorders. BioPsychoSocial Med 7:1
Queiroz LP (2013) Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 17:356
Sleurs D, Tebeka S, Scognamiglio C, Dubertret C, Le Strat Y (2020) Comorbidities of self-reported fibromyalgia in united states adults: a cross-sectional study from the national epidemiological survey on alcohol and related conditions (nesarc-iii). Eur J Pain 24:1471–1483
Velardez-Soto SN, Corral-Frías NS, Amaya MYC, McRae K (2022) Cognitive reappraisal experimental task: replica in mexican university students. Sociedad Española para el Estudio de la Ansiedad y el Estrés 28:30–39
Calandre EP, Rico-Villademoros F, Slim M (2016) Pharmacological treatment of fibromyalgia: is the glass half empty or half full? Pain Manage 7:5–10
Woolfolk RL, Allen LA, Apter JT (2012) Affective-cognitive behavioral therapy for fibromyalgia: a randomized controlled trial. Pain Res Treat 2012:937873–937873
Lumley MA, Schubiner H, Lockhart NA, Kidwell KM, Harte SE, Clauw DJ, Williams DA (2017) Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. Pain 158:2354–2363
Saltychev M, Laimi K (2017) Effectiveness of repetitive transcranial magnetic stimulation in patients with fibromyalgia: a meta-analysis. Int J Rehabil Res 40:11–18
Lloyd DM, Wittkopf PG, Arendsen LJ, Jones AKP (2020) Is transcranial direct current stimulation (tdcs) effective for the treatment of pain in fibromyalgia? A systematic review and meta-analysis. J Pain 21:1085–1100
Balducci T, Rasgado-Toledo J, Valencia A, van Tol M-J, Aleman A, Garza-Villarreal EA (2022) A behavioral and brain imaging dataset with focus on emotion regulation of women with fibromyalgia. Sci Data 9:581
Acknowledgements
We thank Jan-Bernard Marsman and Remco Renken for their assistance in the MRI analysis; Francisco Pellicer Graham, José Sánchez-Sosa, Jorge González-Olvera for their academic assistance; Xochitl Duque for her support to obtain part of the software used [fund CONACYT-FOSISS number 289831]; Raúl Osorio and Lourdes Martínez from the Neuroimaging Department of the National Institute of Psychiatry “Ramón de la Fuente Muñiz”. We are grateful Isabel Barrera and the Mexican Foundation for Fibromyalgia for their help to recruit participants. Finally, and overall we thank our participants. This article is a requirement to T.B. to obtain the grade of Doctor in Science from the Programa de Maestría y Doctorado en Ciencias Médicas y Odontológicas y de la Salud from the National Autonomous University of Mexico.
Funding
Thania Balducci was funded by the National Council of Science and Technology of Mexico (CONACYT) [scholarship number 547524/577656, CVU 547524]; and the University of Groningen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical standards
This study was approved by the Research Ethics Committee of the National Institute of Psychiatry “Ramon de la Fuente Muñiz” in Mexico City, and it was performed in accordance with the ethical standards from the Declaration of Helsinki. All participants gave their informed consent prior to their inclusion in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balducci, T., Garza-Villarreal, E.A., Valencia, A. et al. Abnormal functional neurocircuitry underpinning emotional processing in fibromyalgia. Eur Arch Psychiatry Clin Neurosci 274, 151–164 (2024). https://doi.org/10.1007/s00406-023-01578-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-023-01578-x