Abstract
Illness insight in schizophrenia (SZ) has an important impact on treatment outcome, integration into society and can vary over the course of the disorder. To deal with and treat reduced or absent illness insight, we need to better understand its functional and structural correlates. Previous studies showed regionally abnormal brain volume in brain areas related to cognitive control and self-reference. However, little is known about associations between illness insight and structural and functional network strength in patients with SZ. This study employed a cross-sectional design to examine structural and functional differences between patients with SZ (n = 74) and healthy controls (n = 47) using structural and resting-state functional magnetic resonance imaging (MRI). Voxel-based morphometry was performed on structural data, and the amplitude of low frequency fluctuations (ALFF) was calculated for functional data. To investigate abnormal structure/function interrelationships and their association with illness insight, we used parallel independent component analysis (pICA). Significant group (SZ vs. HC) differences were detected in distinct structural and functional networks, predominantly comprising frontoparietal, temporal and cerebellar regions. Significant associations were found between illness insight and two distinct structural networks comprising frontoparietal (pre- and postcentral gyrus, inferior parietal lobule, thalamus, and precuneus) and posterior cortical regions (cuneus, precuneus, lingual, posterior cingulate, and middle occipital gyrus). Finally, we found a significant relationship between illness insight and functional network comprising temporal regions (superior temporal gyrus). This study suggests that aberrant structural and functional integrity of neural systems subserving cognitive control, memory and self-reference are tightly coupled to illness insight in SZ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia (SZ) is a relatively common psychiatric disorder with an estimated median lifetime prevalence of 0.48% [1,2,3]. SZ poses a large financial burden to society due to medical and socioeconomic costs (e.g., repeated hospitalizations, ambulatory medical care, higher prevalence of cardiovascular comorbidities, ambulatory nursery care, assisted living and unemployment) [4]. One of the core symptoms of SZ is lack of illness insight. Lack of illness insight is associated with higher relapse, increased hospitalization rate, longer duration of hospital stay, higher number of positive and negative symptoms as well as higher risk for suicide and aggressive acts against others [5,6,7,8]. From clinical perspective, illness insight can be divided into two subdomains: (1) clinical insight, describing the awareness of suffering from a disease, recognizing the symptoms related to the disease, associating them with the disease and accepting the necessity of a therapy, (2) cognitive insight, describing the ability to question own perceptions and concepts and if necessary to correct them [9].
In the last two decades, there has been an increase in neuroimaging studies seeking to better understand the neurobiological underpinnings of illness insight in SZ. Studies using magnetic resonance imaging (MRI) found regionally confined alterations in structural networks comprising frontal, parietal, temporal, basal ganglia, and cerebellar regions [9,10,11,12,13,14]. Further, abnormal brain activity at rest has been also related to illness insight in SZ. For instance, Gerretsen and co-workers found associations between symptom insight and self-referential processes ascribed to the so-called default mode network (DMN), together with associations between cognitive insight and the dorsal attention network [15]. In another study of resting-state activity, SZ patients’ samples were assigned to subgroups with good and poor insight, respectively. Special attention was paid to specific DMN subsystems, i.e., anterior and posterior DMN (aDMN, pDMN) [16], i.e., networks that are thought to subserve very distinct mnemonic and self-reflective processes [17]. Although qualitatively different aDMN and pDMN connectivity patterns were found between the patient groups, significant quantitative differences could not be confirmed. However, poor or strong illness insight in SZ patients cannot be explained by only one network alone. It is rather an interplay between different brain regions and networks, which can change their structure and function during the course of the disease. A previous meta-analysis of 21 neuroimaging studies on insight in psychosis conducted by Pijnenborg et al. [9] showed that clinical insight is not associated with abnormalities of isolated brain networks, but with rather diffuse global and frontal abnormalities responsible for cognitive and self-evaluative processes. The situation is different with cognitive insight, which is more dependent on individual brain regions crucial for retrieving and integrating self-related information [18].
It is important to note, that associations with illness insight have been reported for either brain structure or function, with both modalities predominantly assessing brain–behavior relationships on the regional level [9, 19]. Very little is known about associations between illness insight and structural or functional integrity at the neural system level. In addition, aberrant structure–function interrelationships and their effects on specific symptom expression (e.g., hallucinations or sensorimotor symptoms) have been increasingly reported in the past few years [20, 21], yet evidence relating such complex interactions to illness insight is lacking so far. To fill this gap, this study used a multivariate data analysis approach for multimodal data fusion, i.e., parallel independent component analysis (pICA) to identify maximally independent components of each imaging modality as well as the link between them [22, 23]. In particular, we chose amplitude of low frequency fluctuations (ALFF) given its more favorable retest reliability, following the report by Holiga and colleagues in 2018 [24]. While both measures (ALFF and fractional ALFF [fALFF]) exhibited moderate to highest–retest reliability within gray matter regions, reliability for ALFF tended to be higher than for fALFF. At least in part, this difference can be explained by the fact that fALFF is a proportional measure [25]. This finding suggests that ALFF is more reliable than fALFF in gray matter regions, and thus potentially more sensitive for discerning differences between individuals and groups [26]. Furthermore, we used the German version of the Osnabrück scale of adherence and identification of disease-related symptoms in schizophrenia (OSSTI) to measure illness insight [27, 28]. This instrument was chosen because it provides a separate analysis of two distinct dimensions of illness insight, i.e., identification of disease-related symptoms (OSSTI-I) and treatment adherence (OSSTI-A). Further, these two dimensions correspond well with the clinical insight model presented by David et al. [29, 30] and Amador et al. [31], which emphasizes (i) recognition of disease-related symptom as much as (ii) acceptance of treatment necessity [9].
Supporting a multi-dimensional neural model of illness insight influenced by aberrant networks linked to self-perception, self-awareness [32,33,34], and “anosognosia” [35,36,37], we predicted to find interactions between illness insight and both structural and functional network alterations in neural systems subserving cognitive control and self-referential processes.
Methods
Participants
Initially, 133 participants were included in this study. Twelve participants (2 HC, 10 SZ) were excluded during MRI data quality assurance: (a) 4 SZ and 1 HC due to low data quality of the structural MRI data (Participants with 2 or more standard deviations in the Mahalanobis distance were excluded. Mahalanobis distance was used as a measure which combines weighted overall image quality and mean correlation, which represent image artifacts like noise and motion before preprocessing and homogeneity of the data after preprocessing, respectively [38, 39]) and (b) 5 SZ and 1 HC due to excessive head movements (> 3 mm) during resting-state functional magnetic resonance imaging (rs-fMRI)). One hundred and twenty-one participants were considered for further analyses, i.e., 74 patients with SZ and 47 HC.
Recruitment of the participants with SZ took place in the Department of Psychiatry and Psychotherapy at the Central Institute of Mental Health in Mannheim, Germany. This study was conducted as part of a larger project on patients with schizophrenia spectrum disorders (SSD) [40]. As an attempt to minimize clinical heterogeneity and to present this in comparison to other studies on illness insight in SSD patients, we included only patients with a diagnosis of paranoid SZ according to ICD-10 (F20.0). Right-handedness and age between 18 and 65 years were further inclusion criteria. Patients with a history of any substance dependency except for tobacco were excluded. The HCs were recruited via personal communication and community advertisements. In the HC group inclusion criteria were right-handedness and absence of personal or family history of any mental disorder. The study was approved by the local ethics committees (Medical Faculties Mannheim and Heidelberg at Heidelberg University, Germany). Written informed consent was obtained from all participants after a detailed explanation of the aims and procedures of the study.
Clinical assessment
The Positive and Negative Syndrome Scale (PANSS) was used for detailed psychometric assessment of symptoms related to SZ [41]. Participants with SZ were on a stable medication regime for at least 2 weeks, the daily dose of antipsychotic medication was calculated in olanzapine equivalents (OLZe) [42].
The OSSTI was used to measure illness insight in patients [27, 28]. This instrument was developed as a self-rating scale [27, 28], with explicit reference to three pre-existing insight rating instruments, i.e., Scale to Assess Unawareness of Mental Disorder (SUMD) [31], Birchwood's Insight Scale (BIS) [43] and the Self-Appraisal of Illness Questionnaire (SAIQ) [44]. The final version of the OSSTI consists of ten items as 6-point Likert scale, which are divided into two subdomains (OSSTI-A: items: 1, 3, 5, 7, 9, 10, OSSTI-I: items 2, 4, 6, 8, scores of subdomains were used in this study). Higher OSSTI scores refer to higher illness insight. An example for an item of OSSTI-A is: “After discharge of the hospital/ after moving out of the assisted living I will still be in the need of medical (psychiatric) or therapeutic care”. (Item 1) An example for OSSTI-I is: “There exist early warning signs for my mental illness.” (Item 4). Three items (2, 9, 10) are conceptualized with reverse polarity. Waldorf verified the OSSTI in a study on 85 patients with SZ [27]: A Cronbach’s α for standardized items of 0.79 for the whole OSSTI and a mean item intercorrelation of 0.28 was detected. Additionally, a significant correlation with the PANSS item G12 (lack of judgment and insight) of r = 0.54 (p < 0.001) was found. Finally, cognitive functioning was examined via Trail-Making Test B (TMT-B), the Symbol Digit Substitution Test (SDST), and the category fluency (CF) with animals fruits and vegetables as categories [45, 46]. Global functioning was assessed via Global Assessment of Functioning (GAF) [47]. However, these data are available for patients only (see supplementary table 2 for details).
MRI data acquisition
Structural data were acquired using T1-weighted three-dimensional (3D) magnetization-prepared rapid gradient-echo at the Central Institute of Mental Health, Mannheim, Germany on a 3.0 Tesla Magnetom Tim Trio MRI scanner (Siemens Medical Systems) with the following parameters: flip angle 7°, echo time (TE) = 3.93 ms; repetition time (TR) = 2530 ms; inversion time (TI) = 1100 ms; FOV = 256 mm; slice plane = axial; slice thickness: 1 mm; resolution = 1.0 × 1.0 × 1.0 mm; number of slices 176.
For rs-fMRI, 167 whole-brain echo planar imaging (EPI) volumes were recorded in an axial orientation with the following imaging parameters: repetition time = 1790 ms, echo time = 28 ms, field of view = 192 × 192 mm, flip angle = 76°, voxel size = 3 × 3 × 3 mm, 34 slices, slice thickness = 3 mm. For rs-fMRI, all participants were instructed to lay still and with eyes closed, trying not to think of anything specific and neither to fall asleep. Rs-fMRI was followed by immediate verbal contact. A post-scanning exit interview was performed to verify adherence to these instructions.
MRI data analysis
Preprocessing
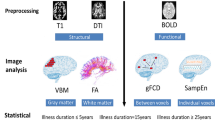
For structural data analyses, the Statistical Parametric Mapping analysis package (SPM12 version 7771; www.fil.ion.ucl.ac.uk/spm/software/spm12/; last access: 27/11/2020) and the computational anatomy toolbox (CAT12 version vcat12.7; dbm.neuro.uni-jena.de/cat/; last access: 27/11/2020) as an extension of SPM12 were used in MATLAB (Version R2019b). Preprocessing included (a) data segmentation and normalization to a template space and segmentation into gray matter, white matter and cerebrospinal fluid, (b) slice display for visual inspection of the images for the manual data quality check, (c) estimation of total intracranial volume (TIV), (d) data quality check, where participants with two or more standard deviations in the Mahalanobis distance were excluded, and (e) smoothing via SPM12: 8 mm Full Width at Half Maximum (FWHM) smoothing was applied.
Preprocessing of resting state-data (rs-data) was performed using the Data Processing Assistant for Resting-State fMRI Advanced Edition (DPARSFA) [48]. For preprocessing of the raw rs-data into mean amplitude of low-frequency fluctuations (mALFF), the following steps were applied in DPARSFA: (a) removing of the first 10 time points (b) slice timing (using the middle slice as reference slice), (c) head motion correction (excessive head motion of > 3 mm or > 3 degrees between two volumes was set as an exclusion criterion, (d) regressing out of the nuisance covariates (mean signals from white matter and cerebrospinal fluid) and movement-related effects using the Friston 24-parameter model [49] and (e) normalization by DARTEL in Montreal Neurological Institute (MNI) space to 3 × 3 × 3 mm3, (e) spatial smoothing with a 6-mm FWHM isotropic Gaussian kernel, and (f) band-pass filtering (0.01–0.1 Hz) to reduce low-frequency drift and high-frequency noise. We chose the mALFF as a parameter for the following statistical analyses representing the rs-fMRI data.
Structural and functional data fusion
For pICA, the Parallel ICA Toolbox (ParaICATv1.0b) in MATLAB R2019b was used (http://trendscenter.org/software/fit). Two features were extracted for the following analysis: structural MRI (sMRI) data as gray matter volume (GMV) and rs-fMRI data as mALFF. Eight independent components (ICs) were estimated for each feature using the minimum description length (MDL). Within the pICA analysis the AA-type was used, which measures the correlation between mixing coefficient of both features. ICASSO was run 20 times to ensure the consistency of the ICs [50]. For visualization of the ICs, the spatial IC-maps of the Parallel ICA Toolbox were put into MRIcroGL v.1.2.20211006 (https://www.nitrc.org/projects/mricrogl) using thresholds of standard deviations |z|> 3.5 and overlay of an MNI-template. The Talairach Daemon database (http://www.talairach.org/daemon.html) was used to extract anatomical labels and stereotaxic coordinates for a threshold of |z|> 3.5 from the spatial IC-maps of the significant ICs of the previous analysis.
Statistical analysis
Following statistical analyses were conducted and displayed offline using the R software environment for statistical computing (version 4.0.3; https://www.r-project.org/; last access: 15/11/2020, [51]). The descriptive figures of the demographics (violin plots) and the correlation graphs were performed using the R package ggplot2 [52]. Between-group differences (HC vs. SZ) were calculated using loading coefficients of sMRI and rs-fMRI ICs. Mann–Whitney-U Test, t test or Welch test (depending on value distribution, as assessed by the Shapiro–Wilk Test) and the homogeneity of variances (Levene test). Following the pathway for partial correlation of the R package ggm [4], two-tailed Pearson’s correlations were used to explore the relationships between OSSTI scores (total, OSSTI-A and OSSTI-I) and loading coefficients of sMRI and rs-fMRI ICs which showed significant between-group differences corrected for multiple comparison (Bonferroni corrected p value of 0.003). Age, sex, total PANSS-score, TIV (only in OSSTI vs GMV) and mean framewise displacement (FD) Power [40] (only in OSSTI vs mALFF) were used as covariates. A nominal significance threshold, a p value < 0.05 was chosen, followed by correction for multiple comparisons using Bonferroni correction (Bonferroni corrected p value of 0.001).
For completeness, we also performed correlation analyses using Spearman’s and Pearson’s correlation to examine the relationship between OSSTI-A, OSSTI-I and PANSS negative score as well as cognitive and global functioning in terms of TMT-B, SDST, CF and GAF scores.
Results
Demographics and clinical scores
Demographic and clinical details are summarized in Table 1. Violin plots with boxplots for mean and SD of OSSTI (OSSTI-total, OSSTI-I, OSSTI-A) and PANSS (total, positive, negative, and general) are shown in Fig. 1 of the supplementary material. There was a significant age difference between the two groups (p ≤ 0.001, Mann–Whitney-U Test) as well as a difference in education years (p < 0.001, Mann–Whitney-U Test). There was no gender difference between the groups.
Group differences
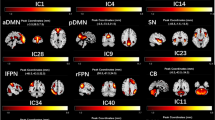
From a total of 8 structural and 8 functional components, we identified 12 networks that differed significantly between SZ patients and HC (p < 0.003, Bonferroni corr.), i.e., seven GMV. For details, see supplementary table 1.
Associations between illness insight and independent components
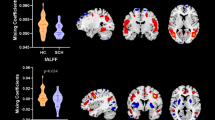
First, we identified a significant negative correlation between the structural frontoparietal network predominantly comprising the postcentral gyrus, inferior parietal lobule (IPL), precentral gyrus (M1), thalamus, precuneus, middle temporal gyrus (MTG), inferior frontal gyrus (IFG), superior temporal gyrus (STG), insula and middle frontal gyrus (MFG) and OSSTI total (OSSTI-total) (– 0.28, p = 0.021) and OSSTI-A (– 0.26, p = 0.028) scores (Figs. 1 and 2, Table 2). Second, we identified a significant negative correlation between structural posterior cortical midline structures comprising the cuneus, precuneus, lingual gyrus, posterior cingulate, and middle occipital gyrus and OSSTI-A scores (– 0.26, p = 0.030) (Figs. 1 and 2, Table 2). Third, the functional medial temporal network predominantly comprising the STG was significantly negatively associated with OSSTI-total (– 0.26, p = 0.027) and OSSTI-I (– 0.26, p = 0.027) scores (Figs. 1 and 2, Table 2). Finally, none of the p values remain significant after the Bonferroni correction (p < 0.001).
Explorative correlation analyses
For completeness, further correlations between illness insight and further structural and functional networks were performed, i.e., for components that did not exhibit significant between-group differences. None of these analyses yielded significant findings. Finally, we did not find any significant associations between OSSTI scores and other clinical and neurocognitive variables (Spearman’s and Pearson’s correlation: all p values > 0.05), except of a correlation between GAF and OSSTI-A (Spearman’s correlation 0.27, p = 0.02).
Discussion
The aim of this multimodal study was to examine the relationship between illness insight and brain structure and resting-state neural activity in SZ patients using pICA. Two main findings emerged: First, we found a significant correlation between illness insight and structural alterations (GMV) of a network comprising frontoparietal and posterior cortical regions. Second, we found a significant relationship between illness insight and medial temporal network activity at rest (mALFF).
First, the frontoparietal network plays an important role in goal-driven behavior, making it possible to react in a fast, accurate and flexible way [53]. The frontoparietal network has often been described to be impaired in SZ patients and hence, there is considerable evidence that frontoparietal dysfunction in SZ patients is also associated with negative symptoms and cognitive impairment [18, 54, 55]. Interestingly, previous sMRI studies using voxel-based morphometry (VBM) and cortical thickness found an association between the right dorsolateral prefrontal cortex (DLPFC), other prefrontal regions and illness insight in SZ [19, 56, 57]. This said, structural alterations of the frontoparietal network in SZ may not only lead to negative symptoms and cognitive deficits, but to impaired illness insight as well.
Second, we found a significant association between functional alterations of the medial temporal network and OSSTI total and OSSTI-I scores. This finding is noteworthy, because the medial temporal network is a subsystem of the DMN and has been shown to play an important role in memory retrieval and prospection [58,59,60]. Alterations in this network could cause difficulties for SZ patients when they are overthinking their thoughts, perceptions, and actions, and try to put them into the context of their past, presence, and future experiences. This said, SZ patients have less abilities to put their own thoughts and perceptions into the environmental context and are therefore not able to identify them correctly as a part of their psychotic experience.
Third, with the posterior cortical midline structures we identified a significant relationship between functional alterations of the posterior DMN (pDMN) and varying illness insight. PDMN is the second subsystem of the DMN and is involved in self-representation, emotion, and salience detection [61]. This finding is also in line with the study of Liemburg et al. [16] who found a disconnection between the anterior and posterior DMN in SZ patients with poor illness insight. However, these results did not reach statistical significance [16]. Interestingly, although for obvious reasons it is difficult to directly compare structural with functional MRI studies, previous sMRI studies performing VBM and surface-based morphometry (SBM) analyses found structural alterations in different regions belonging to this network especially in the precuneus and poor illness insight [9]. Overall, the inability to remember previous episodes (due to disturbances of the medial temporal network) and to correctly assess current symptoms (due to disturbances of the pDMN) might lead to reduced illness insight and related consequences.
Interestingly, we found an association between treatment adherence (OSSTI-A) and structural changes as well as disease-related symptoms (OSSTI-I) and functional changes. Because no neuroimaging studies to date have investigated the neuronal correlates in such a differentiated way, we might only speculate about the pathomechanism underlying these aspects. On one side, psychopathological symptoms and the patient’s ability to classify his/her symptoms as part of the disease can often fluctuate along the disease course. This can then be possibly reflected in the aberrant function of different brain networks. On the other side, therapeutic adherence is a more stable/persistent feature in patients with partial or full remission and therefore more attributable to structural and spatially more delineated changes of particular brain networks. The review and meta-analysis by Pijnenborg et al. [9] was also able to show that different forms of insight (clinical and cognitive) have different neuronal correlates. Pijnenborg et al. [9] showed that clinical insight is related to spatially diffuse abnormalities across the brain; whereas, cognitive insight is mainly associated with ventrolateral prefrontal cortex and hippocampal areas. Finally, the absence of significant correlations between OSSTI scores and neurocognitive variables is noteworthy, since it supports a model where illness insight is conceived as a distinct symptom domain that doesn’t entirely overlap with the cognitive domain.
The strengths of this study consist of the large sample size and the use of pICA for the analysis of structural and functional MRI data. This method makes it possible to outline specific networks individually and to combine rs-fMRI and sMRI data analyses. However, this study also has limitations: (a) The cross-sectional study design is a limitation, because clinical disease course, psychopathology, and illness insight may fluctuate over time. This said, a cross-sectional study design is able to capture only one specific moment of illness insight. Illness insight at this particular time point can be modulated by situational effects that are affected by different psychopathological symptoms. To minimize situational effects potentially driven by overall symptom severity, we used the PANSS total score as a covariate. In future studies, situational effects may be better counteracted by more fine-grained methodological approaches, e.g., ecological momentary assessments (EMA). Such techniques may be better suited to detect fluctuations within longer time periods. In addition, in the context of this study functional neuroimaging markers are temporally less stable than structural parameters. Still, this study can provide initial indications of structural and functional network changes underlying different domains of illness insight in SZ. Our results are also consistent with our initial hypotheses and current literature that aspects of illness insight, which are of different temporal stability (recognition of psychopathological symptoms and acceptance of therapy), are associated with different neuroimaging parameters. (b) Since we compared SZ patients with HC, we may have identified SZ-associated brain changes rather than the true neuronal correlates of illness insight. Therefore, we strongly acknowledge MRI studies comparing SZ patients with and without illness insight to identify true neuronal correlates of illness insight. (c) The comparison with previous MRI studies on illness insight is difficult because of the usage of different scales for the measurement of illness insight and MRI analysis methods. Therefore, it is important to form national and international collaborations and to use standardized protocols when examining neuronal correlates underlying illness insight in SZ and other psychiatric disorders. (d) The identified association between the network strength and OSSTI scores did not survive Bonferroni correction for multiple testing. While these associations may appear neurobiologically plausible, and consistent with previous MRI studies on insight in SZ [62,63,64], such findings need to be interpreted with appropriate caution.
Conclusion
Illness insight is associated with aberrant structural and functional networks responsible for self-reflection, memory, and memory retrieval as well as internal mentation. These findings may yield valuable translational potential, since targeting such network dysfunctions, e.g., via specific psychotherapeutic interventions or non-invasive neuromodulation may well lead to improved illness insight in SZ and subsequently to more favorable clinical and psychosocial outcomes [65].
Availability of data and materials
Data will be made available upon request to the extent permitted by the requirements of the European General Data Protection Regulation (GDPR).
Code availability
Codes will be made available upon scientifically reasonable request.
Abbreviations
- BIS:
-
Birchwood’s Insight Scale
- CF:
-
Category fluency
- DLPFC:
-
Dorsolateral prefrontal cortex
- EPI:
-
Echo planar imaging
- GAF:
-
Global Assessment of Functioning
- ICs:
-
Independent components
- MFG:
-
Middle frontal gyrus
- MRI:
-
Magnetic resonance imaging
- sMRI:
-
Structural MRI
- rs-fMRI:
-
Resting-state functional MRI
- M1:
-
Precentral gyrus
- DK40:
-
Desikan–Killiany atlas
- DMN:
-
Default mode network
- aDMN:
-
Anterior DMN
- pDMN:
-
Posterior DMN
- FDR:
-
False Discovery Rate
- FOV:
-
Field of view
- FD:
-
Framewise displacement
- FDR:
-
False discovery rate
- FWHM:
-
Full width at half maximum
- HC:
-
Healthy controls
- IFG:
-
Inferior frontal gyrus
- IPL:
-
Inferior parietal lobule
- mALFF:
-
Mean amplitude of low frequency fluctuations
- MDL:
-
Minimum description length
- MNI:
-
Montreal neurological institute
- MTG:
-
Middle temporal gyrus
- OLZe:
-
Olanzapine equivalents
- OSSTI:
-
Osnabrück scale of adherence and identification of disease-related symptoms by schizophrenia (originally in German: Osnabrücker Skala zu Therapieeinstellung und Identifikation psychischer Beschwerden bei Schizophrenie)
- OSSTI-A:
-
Adherence domain of OSSTI
- OSSTI-I:
-
Identification of disease-related symptoms domain of OSSTI
- OSSTI-total:
-
Total score of the OSSTI
- PANSS:
-
Positive and Negative Syndrome Scale
- pICA:
-
Parallel independent component analysis
- ROI:
-
Region of interest
- rs:
-
Resting state
- SAIQ:
-
Self-Appraisal of Illness Questionnaire
- SBM:
-
Surface-based morphometry
- SD:
-
Standard deviation
- SDST:
-
Symbol Digit Substitution Test
- SPM12:
-
Statistical Parametric Mapping
- STG:
-
Superior temporal gyrus
- SUMD:
-
Scale to Assess Unawareness of Mental Disorder
- SZ:
-
Patients with schizophrenia
- TE:
-
Echo time
- TI:
-
Inversion time
- TIV:
-
Total intracranial volume
- TMT-B:
-
Trail-Making Test B
- TR:
-
Repetition time
- VBM:
-
Voxel-based morphometry
References
McGrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76
Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R (2015) An evaluation of variation in published estimates of schizophrenia prevalence from 1990–2013: a systematic literature review. BMC Psychiatry 15:1–14
Baba K, Guo W, Chen Y, Nosaka T, Kato T (2022) Burden of schizophrenia among Japanese patients: a cross-sectional national health and wellness survey. BMC Psychiatry 22:1–14
Jauhar S, Johnstone M, McKenna PJ (2022) Schizophrenia. The Lancet 399:473–486
Baier M (2010) Insight in schizophrenia: a review. Curr Psychiatry Rep 12:356–361
Buckley PF, Hrouda DR, Friedman L, Noffsinger SG, Resnick PJ, Camlin-Shingler K (2004) Insight and its relationship to violent behavior in patients with schizophrenia. Am J Psychiatry 161:1712–1714
Lysaker PH, Pattison ML, Leonhardt BL, Phelps S, Vohs JL (2018) Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry 17:12–23
Robinson D, Woerner MG, Alvir JMJ, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D (1999) Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 56:241–247
Pijnenborg G, Larabi D, Xu P, Hasson-Ohayon I, de Vos A, Ćurčić-Blake B, Aleman A, Van der Meer L (2020) Brain areas associated with clinical and cognitive insight in psychotic disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev 116:301–336
Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY, Kim SI, Kwon JS (2004) Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res: Neuroimaging 132:251–260
Bassitt DP, Neto MR, de Castro CC, Busatto GF (2007) Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci 257:58–62
Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O (2011) Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand 123:431–439
McFarland J, Cannon DM, Schmidt H, Ahmed M, Hehir S, Emsell L, Barker G, McCarthy P, Elliott MA, McDonald C (2013) Association of grey matter volume deviation with insight impairment in first-episode affective and non-affective psychosis. Eur Arch Psychiatry Clin Neurosci 263:133–141
Sapara A, Ffytche DH, Cooke MA, Williams SC, Kumari V (2016) Voxel-based magnetic resonance imaging investigation of poor and preserved clinical insight in people with schizophrenia. World J Psychiatry 6:311
Gerretsen P, Menon M, Mamo DC, Fervaha G, Remington G, Pollock BG, Graff-Guerrero A (2014) Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr Res 160:43–50
Liemburg EJ, van der Meer L, Swart M, Curcic-Blake B, Bruggeman R, Knegtering H, Aleman A (2012) Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PLOS ONE 7:e42707
Fahmy R, Wasfi M, Mamdouh R, Moussa K, Wahba A, Schmitgen MM, Kubera KM, Wolf ND, Sambataro F, Wolf RC (2019) Mindfulness-based therapy modulates default-mode network connectivity in patients with opioid dependence. Eur Neuropsychopharmacol 29:662–671
Galderisi S, Merlotti E, Mucci A (2015) Neurobiological background of negative symptoms. Eur Arch Psychiatry Clin Neurosci 265:543–558
Otte M-L, Schmitgen MM, Kubera KM, Wolf ND, Fritze S, Geiger LS, Tost H, Seidl UW, Meyer-Lindenberg A, Hirjak D (2021) Cortical morphology and illness insight in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci pp. 1–11
Kubera KM, Rashidi M, Schmitgen MM, Barth A, Hirjak D, Sambataro F, Calhoun VD, Wolf RC (2019) Structure/function interrelationships in patients with schizophrenia who have persistent auditory verbal hallucinations: a multimodal MRI study using parallel ICA. Prog Neuropsychopharmacol Biol Psychiatry 93:114–121
Wolf RC, Rashidi M, Fritze S, Kubera KM, Northoff G, Sambataro F, Calhoun VD, Geiger-Primo LS, Tost H, Hirjak D (2020) A neural signature of parkinsonism in patients with schizophrenia spectrum disorders. Schizophr Res 231:54–60
Sui J, Adali T, Yu Q, Chen J, Calhoun VD (2012) A review of multivariate methods for multimodal fusion of brain imaging data. J Neurosci Methods 204:68–81
Liu J, Demirci O, Calhoun VD (2008) A parallel independent component analysis approach to investigate genomic influence on brain function. IEEE Signal Process Lett 15:413–416
Holiga Š, Sambataro F, Luzy C, Greig G, Sarkar N, Renken RJ, Marsman J-BC, Schobel SA, Bertolino A, Dukart J (2018) Test-retest reliability of task-based and resting-state blood oxygen level dependence and cerebral blood flow measures. PLoS ONE 13:e0206583
Arndt S, Cohen G, Alliger RJ, Swayze VW II, Andreasen NC (1991) Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res: Neuroimaging 40:79–89
Zuo X-N, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP (2010) Reliable intrinsic connectivity networks: test–retest evaluation using ICA and dual regression approach. Neuroimage 49:2163–2177
Waldorf M (2010) Krankheitseinsicht, dynamisch getestete exekutivfunktionen und defensive bewältigung bei schizophrenie. Unveröffentlichte Dissertation, Universität Osnabrück
Krupa T (2005) Krankheitseinsicht, copingstrategien und neurokognitive beeinträchtigungen bei schizophrenen patienten. Unveröffentlichte Diplomarbeit, Universität Osnabrück
David AS (1990) Insight and psychosis. Br J Psychiatry: J Mental Sci 156:798–808
David AS (1990) On insight and psychosis: discussion paper. J R Soc Med 83:325–329
Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM (1993) Assessment of insight in psychosis. Am J Psychiatry 150:873–873
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447
Whitfield-Gabrieli S, Ford JM (2012) Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76
Goldberg II, Harel M, Malach R (2006) When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron 50:329–339
Pia L, Neppi-Modona M, Ricci R, Berti A (2004) The anatomy of anosognosia for hemiplegia: a meta-analysis. Cortex 40:367–377
McGlynn SM, Schacter DL (1989) Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol 11:143–205
Turnbull OH, Fotopoulou A, Solms M (2014) Anosognosia as motivated unawareness: the ‘defence’hypothesis revisited. Cortex 61:18–29
Mahone EM, Puts NA, Edden RAE, Ryan M, Singer HS (2018) Gaba and glutamate in children with tourette syndrome: a (1)h mr spectroscopy study at 7t. Psychiatry Res Neuroimaging 273:46–53
Gaser C, Kurth F (2017) Manual computational anatomy toolbox-cat12. Version 03.07.2017. Structural brain mapping Group at the Departments of Psychiatry and Neurology, University of Jena 69
Fritze S, Sambataro F, Kubera KM, Brandt GA, Meyer-Lindenberg A, Wolf RC, Hirjak D (2022) Characterizing the sensorimotor domain in schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci 272:1097–1108
Kay SR, Opler LA, Lindenmayer J-P (1989) The positive and negative syndrome scale (panss): rationale and standardisation. Br J Psychiatry 155:59–65
Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, Geddes J, Davis JM (2015) Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull 41:1397–1402
Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M (1994) A self-report insight scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand 89:62–67
Marks KA, Fastenau PS, Lysaker PH, Bond GR (2000) Self-appraisal of illness questionnaire (SAIQ): relationship to researcher-rated insight and neuropsychological function in schizophrenia. Schizophr Res 45:203–211
Cuesta MJ, Pino O, Guilera G, Rojo JE, Gomez-Benito J, Purdon SE, Franco M, Martinez-Aran A, Segarra N, Tabares-Seisdedos R, Vieta E, Bernardo M, Crespo-Facorro B, Mesa F, Rejas J (2011) Brief cognitive assessment instruments in schizophrenia and bipolar patients, and healthy control subjects: a comparison study between the brief cognitive assessment tool for schizophrenia (b-cats) and the screen for cognitive impairment in psychiatry (scip). Schizophr Res 130:137–142
Hurford IM, Marder SR, Keefe RS, Reise SP, Bilder RM (2011) A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull 37(3):538–545. https://doi.org/10.1093/schbul/sbp095
DSM-III.R. DKuDddusMpSr (1989) Gaf-skala: GLOBAL assessment of functioning scale. In: Beltz, Weinheim, Basel
Yan C-G, Wang X-D, Zuo X-N, Zang Y-F (2016) Dpabi: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14:339–351
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996) Movement-related effects in FMRI time-series. Magn Reson Med 35:346–355
Himberg J, Hyvärinen A, Esposito F (2004) Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22:1214–1222
Team RC (2020) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria.
Wickham H (2016) Ggplot2: Elegant graphics for data analysis. Springer, New York
Marek S, Dosenbach NU (2022) The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci
Brandl F, Avram M, Weise B, Shang J, Simões B, Bertram T, Ayala DH, Penzel N, Gürsel DA, Bäuml J (2019) Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biol Psychiat 85:573–583
Dong D, Wang Y, Chang X, Luo C, Yao D (2018) Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull 44:168–181
Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS (2004) Insight and prefrontal cortex in first-episode schizophrenia. Neuroimage 22:1315–1320
Buchy L, Ad-Dab’bagh Y, Lepage C, Malla A, Joober R, Evans A, Lepage M (2012) Symptom attribution in first episode psychosis: A cortical thickness study. Psychiatry Res: Neuroimaging 203:6–13
Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL (2010) Evidence for the default network’s role in spontaneous cognition. J Neurophysiol 104:322–335
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38
Kim H (2021) Imaging recollection, familiarity, and novelty in the frontoparietal control and default mode networks and the anterior-posterior medial temporal lobe: an integrated view and meta-analysis. Neurosci Biobehav Rev 126:491–508
Vogt BA, Vogt L, Laureys S (2006) Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 29:452–466
Asmal L, du Plessis S, Vink M, Fouche JP, Chiliza B, Emsley R (2017) Insight and white matter fractional anisotropy in first-episode schizophrenia. Schizophr Res 183:88–94
Sapara A, Ffytche DH, Cooke MA, Williams SC, Kumari V (2016) Voxel-based magnetic resonance imaging investigation of poor and preserved clinical insight in people with schizophrenia. World J Psychiatry 6:311–321
Curcic-Blake B, van der Meer L, Pijnenborg GH, David AS, Aleman A (2015) Insight and psychosis: functional and anatomical brain connectivity and self-reflection in schizophrenia. Hum Brain Mapp 36:4859–4868
Blay M, Adam O, Bation R, Galvao F, Brunelin J, Mondino M (2022) Improvement of insight with non-invasive brain stimulation in patients with schizophrenia: a systematic review. J Clin Med 11:40
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Research Foundation (DFG) (grant number DFG HI 1928/2-1 and HI 1928/6-1 to D.H. and WO 1883/6-1 and WO 1883/15-1 to R.C.W. and EB 187/8-1 to S.F.) and German Federal Ministry of Education and Research (BMBF, grant 01GQ1102 to H.T.) as well as National Institute of Health grant #R01MH118695 (to V.D.C.) and National Science Foundation grant #2112455 (to V.D.C). The funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
DH, UWS and RCW designed the study. DH, RCW, HT and VDC obtained funding. DH, SF, LSG, and HT recruited, assessed, and scanned subjects. M-LO, MMS and RCW performed the analysis. RCW, MMS and VDC supervised analyses. NDW, KMK, and AM-L interpreted and critically discussed the results. M-LO, RCW and DH wrote the first draft of the manuscript. All authors contributed to and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Ethical approval
All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised 2008. The local ethics committees (Medical Faculties Heidelberg and Mannheim at Heidelberg University, Germany) approved the study. Written informed consent was obtained from all participants following a complete description of the study.
Consent for publication
All patients gave informed consent to participate in the study and for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otte, ML., Schmitgen, M.M., Wolf, N.D. et al. Structure/function interrelationships and illness insight in patients with schizophrenia: a multimodal MRI data fusion study. Eur Arch Psychiatry Clin Neurosci 273, 1703–1713 (2023). https://doi.org/10.1007/s00406-023-01566-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-023-01566-1