Abstract
Epidemiological studies have shown that gestational age and birth weight are linked to cognitive performance in adults. On a neurobiological level, this effect is hypothesized to be related to cortical gyrification, which is determined primarily during fetal development. The relationships between gestational age, gyrification and specific cognitive abilities in adults are still poorly understood. In 542 healthy participants, gyrification indices were calculated from structural magnetic resonance imaging T1 data at 3 T using CAT12. After applying a battery of neuropsychological tests, neuropsychological factors were extracted with a factor analysis. We conducted regressions to test associations between gyrification and gestational age as well as birth weight. Moderation analyses explored the relationships between gestational age, gyrification and neuropsychological factors. Gestational age is significantly positively associated with cortical folding in the left supramarginal, bilaterally in the superior frontal and the lingual cortex. We extracted two neuropsychological factors that describe language abilities and working memory/attention. The association between gyrification in the left superior frontal gyrus and working memory/attention was moderated by gestational age. Further, the association between gyrification in the left supramarginal cortex and both, working memory/attention as well as language, were moderated by gestational age. Gyrification is associated with gestational age and related to specific neuropsychological outcomes in healthy adulthood. Implications from these findings for the cortical neurodevelopment of cognitive domains and mental health are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiological studies have shown that aspects of fetal growth such as birth weight and gestational age are positively correlated with cognitive abilities in adults [1,2,3,4,5,6]. The brain morphological mechanisms underlying these effects are not yet fully understood.
The fetus is constantly exposed to environmental effects [7]. For example, maternal stress [8, 9], anxiety [10, 11], smoking [12], malnutrition [13] and social status [14] are associated with lower birth weight and gestational age. Therefore, birth weight and gestational age served as surrogate markers for a large variety of environmental influences on prenatal development in many studies [1].
Gyrification is an index measuring cortical folding which can be extracted from MRI images. This measure has been utilized in imaging studies investigating the course of cortical development in utero, in new-borns, and throughout life [15, 16]. Reduced birth weight or shortened gestational age leads to increases and decreases in gyrification distributed over large parts of the cortex, but especially in fronto-temporo-parietal regions [17, 18]. Consistent with the current conceptualization of gyrification as an index largely determined during intrauterine development [19,20,21], these structural alterations persist over the whole life span and can still be found in adults. Further, some of these gyrification alterations associated with prenatal growth have been linked to neurocognitive performance in infants and adults [17, 18, 22]: Full-scale IQ has been associated with the left fusiform gyrus and lateral orbitofrontal cortex, the right superior parietal gyrus [18], the bihemispheric lateral and anterior temporal cortices and the occipitotemporal junction [17]. Regional variations in gyrification have repeatedly been associated with general neurocognitive performance in humans [23, 24] and are considered as a potential neurocellular correlate of cognitive abilities.
While these studies have advanced our knowledge considerably, they leave several questions unanswered: 1. Many of the previous studies used only group comparisons (with arbitrary criteria for the definition of cut-offs for group divisions that were not based on neurobiological measures), rather than dimensional approaches, resulting in a loss of variance. 2. Most studies used only moderate sample sizes leading to little statistical power and limited generalizability. 3. Cortical folding takes place in a non-linear course primarily during the second half of intrauterine development but also non-linearly ex utero in infants [1, 25,26,27]. However, previous studies did not test for non-linear associations between variables of prenatal growth and brain morphology. 4. Some studies included participants with adverse events during their gestation such as birth complications, maternal infection, alcohol or drug abuse, medication, or maternal malnutrition [15, 17, 18, 28, 29], which all confound birth weight/gestational age as well as brain structural alterations. 5. Lastly, most studies investigated only intelligence by using abbreviated tests and refrained from measuring a broad range of cognitive domains. Some also used merely subjective parental reports as a proxy of child cognitive abilities. As a result, effects on specific cognitive domains, e.g., working, short- and long-term memory, attention or language have yet to be investigated.
In our study, we aimed to address these points. We recruited a large sample of healthy participants but excluded all subjects born after a high-risk pregnancy who had been exposed to gross harmful environmental influences, e.g., maternal infections, drug use, malnutrition or birth complications. Further, we used a comprehensive neuropsychological test battery to investigate associations between gyrification, fetal growth and specific neuropsychological domains. We hypothesized: 1. Gestational age as well as 2. birth weight are associated with reduced gyrification in healthy adults. 3. These relationships are non-linear. 4. Finally, we test whether gyrification is related to neuropsychological performance in adults, thus bridging the explanatory gap between prenatal development and adult neuropsychological outcomes.
Materials and methods
Sample
Healthy participants were recruited from the ongoing bicentric FOR2107 study (http://for2107.de/; [30]). All subjects underwent the Structured Clinical Interview (SCID-I; [31]) based on the DSM-IV-TR which was administered by trained researchers to ensure absence of current or history of any psychiatric disorders over life time. Additional exclusion criteria were an IQ < 80 (estimated with the MWT-B [32], a German equivalent of the National Adult Reading Test (NART; [33])) or age > 65 years or < 18 years. Further, participants with current or previous substance dependence, major medical conditions (e.g., cancer, chronic autoimmune diseases, infections) and any history of neurological diagnoses (stroke, tumor, neuro-inflammatory diseases, epilepsy, head-trauma) were excluded. All participants with contraindications to MRI (e.g., pregnancy, ferromagnetic implants, claustrophobia), were also excluded from this study.
Based on the literature, we identified different types of high-risk pregnancies that could confound our results and excluded all subjects born after such high-risk pregnancies. These risk factors were: maternal infections which are associated with increased chance for long-term cognitive deficits [34] and mental retardation [35] and alcohol consumption by the mother during pregnancy, which is associated with behavioral problems, cognitive deficits and increased stress reactivity [36,37,38]. Additionally, we excluded participants whose mothers used drugs during pregnancy which is also associated with higher risk for behavioral problems and cognitive deficits [39,40,41,42,43]. Another exclusion criterion was maternal malnourishment which substantially affects fetal brain development [15, 44] that may have long-term consequences for behavioral problems and cognitive function later in life [45,46,47,48,49]. Multiple births were also excluded because fetal growth restrictions are associated with a decrease in cortical folding [50, 51], which is more common in multiple pregnancies [52]. Last, subjects who were born with severe obstetric complications were also excluded, e.g., ischemia, which is associated with an increase for both mental disorders [53, 54] as well as brain structural alterations in cortical volumes and total surface areas [55] and a decrease in intelligence [56]. Detailed information on the course of pregnancy and the presence of a high-risk pregnancy, including gestational age and birthweight, were obtained via questionnaires. These were sent to the participants via mail two weeks before the appointment for MRI examination, so that they had enough time to obtain this information, e.g., from their birth certificate.
The final sample consisted of 542 nonclinical participants. Their birth weight ranged from 1000 to 5300 g, their gestational age from 28 to 42 weeks. Birth weight and gestational age were correlated (r = 0.378; p < 0.001). Based on the WHO definition of preterm birth (being born before the 38th week of gestation [57]), 51 subjects from the 542 participants were born premature. 367 participants (67.7%) were female, the mean age of the sample was 31.6 years (SD = 11.5 years) and, on average, participants had 13.93 years of education (SD = 2.4). Characteristics of participants are listed in Table 1.
MRI data acquisition
We acquired MRI data at two sites and in accordance with our quality assurance protocol [58]. In Marburg, the MRI data were acquired with a 3 T MRI scanner (Tim Trio, Siemens, Erlangen, Germany) using a 12-channel head matrix Rx-coil. In Münster, a 3 T MRI scanner (Prisma, Siemens, Erlangen, Germany) and a 20-channel head matrix Rx-coil were used. Parameters of the MP-RAGE sequences differed slightly across sites (Münster: TR = 1900 ms, TE = 2.28 ms, Flip angle = 8°, 192 sagittal slices, voxel size 1 × 1x1 mm, acquisition duration = 4:58 min; Marburg: TR = 1900 ms, TE = 2.26 ms, Flip angle = 9°, 176 sagittal slices, voxel size 1 × 1 × 1 mm, acquisition duration = 4:26 min). Precluding their further preprocessing, scans were manually checked for the absence of artifacts and inspected for anatomical abnormalities by a senior clinician (UD). In doing so, we excluded scans from six participants.
MRI data preprocessing and statistical analysis of gyrification data
For surface based morphometry analyses, we used the CAT12 toolbox (version r1450) that builds on SPM [59]. Applying default settings, we extracted cortical surfaces using a spherical harmonics approach [60], applied topological correction [61] and mapped surfaces spherically with an adopted volume-based diffeomorphic DARTEL algorithm [62] to reparametrize them into a common coordinate system to allow inter-subject comparisons [63]. We then estimated cortical gyrification utilizing an approach based on local cortical absolute mean curvature (AMC; [64]). Increases in the amplitude and frequency of the folding of the cortex are reflected in increased values of smoothed absolute mean curvature. All modulated gyrification data sets were smoothed with a 25 mm full width at half maximum Gaussian kernel. For cluster labeling, we chose the Desikan-Killiany-40 Atlas [65].
Separate polynomial general linear models were calculated with gestational age (in weeks) and birth weight, respectively, as regressors and gyrification as regressand. To investigate potential quadratic functions, we used the CAT12 function cat_stat_polynomial to estimate quadratic functions of the parameters birth weight and gestational age. Additionally, we used age, gender, site and scanner as covariates. Further, we conducted two-sample t-tests comparing participants born after full-term pregnancy versus participants born preterm regarding differences in gyrification with identical covariates.
Contrasts were processed using the TFCE Toolbox (threshold-free cluster enhancement; version r211) according to the Smith Method and with 8000 permutations [66,67,68]. We set significance levels at α < 0.05, family wise error corrected (FWE; [69, 70]). FWE-significant mean cluster raw values were extracted for each participant for further analyses (see below).
Neuropsychological assessment
The neuropsychological assessment took about 50 min and contained eight different tests that measure verbal fluency (Regensburger Wortflüssigkeits-Test (RWT); [71]), verbal IQ (Mehrfachwahl-Wortschatztest (MWT-B); [32]), processing speed (Trail Making Test (TMT-B); 72, digit symbol substitution test (DSST); [73]), sustained-attention (sustained-attention test d2; [74]), declarative short- and declarative long-term memory (Verbaler Lern- und Merkfähigkeitstest (VLMT-A and VLMT-B; [75])) and working memory (Letter–number sequencing subtest (LNST; 73, Corsi; [76])). Complete neuropsychological data were available for 530 participants. For further information on the execution of the neuropsychological assessment and an overview of used test scores, see supplement; for a descriptive statistics of neuropsychological test scores, see Table 1.
We conducted an exploratory factor analysis with SPSS 24 [77] using the aforementioned neuropsychological test scores to describe variability among inter-correlated variables and generate a set of factors with reduced dimensionality. Factors were extracted by principal axis factoring and number of factors was determined using a scree test [78]. Maximizing loadings of variables on particular factors was ensured by conducting a varimax rotation. The Kaiser–Meyer–Olkin measure of sampling was 0.846, indicating the test measures sampling adequacy is given. Barlett’s test of Sphericity yielded a significant result (p < 0.001) suggesting that correlations between items were sufficiently large for performing a factor analysis. An anti-image correlation matrix revealed that all variables showed MSA-values (measure of sampling adequacy) > 0.7, implicating that they are all suitable for being used in a factor analysis (Table S1). Neuropsychological test scores intercorrelations revealed that none of them showed intercorrelations with the other variables always below 0.3, meaning that we did not include variables merely contributing to the understanding of the factor’s underlying data structure (Table S2). Additionally, no intercorrelation was above 0.9 suggesting no existence of multicollinearity (Table S2). For each subject, factor scores were computed utilizing a regression approach.
Partial correlations between neuropsychological factors and gyrification
To investigate potential relationships between extracted mean gyrification from significant clusters and neuropsychological test outcome, we performed partial correlations while controlling for years of education. Brain structural alterations are much more pronounced in humans born premature [26, 79,80,81,82]. Therefore, we performed partial correlations separating participants into two groups: born preterm (as defined as gestational age shorter than 38 weeks by the WHO [57]) and born from the 38th week of gestation or later.
Moderation analyses
We conducted moderation analyses using the PROCESS macro 3.5 [83] available for SPSS to investigate contributions of gestational age on the gyrification–gestational age associations and neuropsychological performances. Raw mean gyrification values were extracted from significant clusters of vertices and subsequently used as our predictors, the two extracted neuropsychological factors as outcome variables, gestational age in weeks as moderator and, additionally, years of education [84] as a covariate. Heteroscedasticity consistent standard error and covariance matrix estimator was used (HC3) [85, 86] and continuous variables that define products were mean-centered prior to analysis. Due to the negative skewness of the variable gestational age (skewness = − 2) moderations were probed at the 16th, 50th and 84th percentiles.
Ethics
The FOR2107 cohort project was approved by the Ethics Committees of the Medical Faculties, University of Marburg (AZ: 07/14) and University of Münster (AZ: 2014-422-b-S). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All subjects gave written informed consent to our study protocol and received financial compensation for their expense.
Results
Gyrification
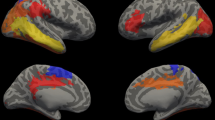
We found significant positive linear associations between gestational age (mean = 39.57 weeks; SD = 1.63 weeks) and five gyrification clusters (s. Fig. 1, Table 2 and Fig. S1 for scatter plots) mainly involving the left supramarginal cortex (k = 881, TFCE = 12,398.62, pFWE = 0.036), the left (k = 1978, TFCE = 13,555.62, pFWE = 0.028) and right superior frontal cortex (k = 566, TFCE = 15,217.21, pFWE = 0.019), and both the left (k = 1786, TFCE = 17,795.10, pFWE = 0.01) and right lingual cortex (k = 671, TFCE = 12,027.61, pFWE = 0.04). We found neither negative linear nor positive and negative quadratic associations between duration of pregnancy and gyrification. Conducted t-tests showed similar results. Participants born preterm showed relatively less gyrification compared to those born after full-term pregnancy in two significant clusters (k = 1057, TFCE = 15,713.68, pFWE = 0.014; k = 7591, TFCE = 15,199.91, pFWE = 0.016) that again included the bilateral superior frontal cortex, the left supramarginal cortex but not the lingual cortex (see Table S3 and Fig. S2). There were no brain areas in which participants born preterm showed significant more gyrification compared to participants born after full-term pregnancy. Birth weight (mean = 3416 g; SD = 528.2 g) was not significantly associated with gyrification in any of the regressions.
Associations between gyrification and gestational age. Statistical parametric map of positive associations between gestational age (in weeks) and gyrification in a multiple regression. Threshold-free cluster enhancement was used at a threshold of p < 0.05 (FWE-corrected). Warmer colors represent lower p values
Factor analysis of neuropsychological test scores
Based on the “elbow criterion” of a scree test (see Fig. S3) we retained two factors [78] that explained in total 36.2% of the variance in the neuropsychological data. The first one is mainly comprising neuropsychological tests measuring working memory and attention (eigenvalue = 3.46; with high rotated factor loadings on d2-KL, TMT-B and Corsi task; rotated sum of squared factor loadings explaining 17.56% variance), the second one consists mainly of language performance (eigenvalue = 1.32; highest rotated factor loadings on all subtests of the RWT and the MWTB; rotated sum of squared factor loadings explaining 10.82% variance). All rotated factor loadings on factor attention/working memory and factor language are shown in Table S4.
Bivariate partial correlations gyrification and neuropsychological factors
We found several significant correlations between the extracted neuropsychological factors and mean gyrification cluster values from the conducted multiple regression (Table 3). In particular, the right superior frontal gyrus was related to language in the whole sample and the left supramarginal gyrus to both, working memory and language in the preterm subsample (28–38 weeks), among others. In this partial correlation analysis, gestational age in weeks was not associated with the neuropsychological factors (Table 3). Correlations between the neuropsychological factors gained from the factor analysis and gyrification clusters from t-tests showed similar results and are provided in the supplement (Table S5).
Moderation analysis
In three models, gestational age significantly moderated the association between mean gyrification cluster values extracted from significant clusters in the multiple regressions and neuropsychological factors while controlling for years of education (s. Fig. 2 and Table 4). Gestational age significantly moderated the association between the left supramarginal gyrification cluster and the factor working memory/attention (model: F(4/525) = 4.886, p = 0.001, R2 = 3.4%; moderation: t = − 2, p = 0.047, R2(change) = 0.8%) as well as the association between the same gyrification cluster and the factor language (model: F(4/525) = 4.015, p = 0.003, R2 = 3.1%; moderation: t = − 2.19, p = 0.029, R2(change) = 1%). Additionally, the association between the left superior frontal gyrification cluster and the factor working memory/attention was significantly moderated by gestational age (model: F(4/525) = 4.892, p = 0.001, R2 = 3.4%; moderation: t = − 2.04, p = 0.041, R2(change) = 0.7%). In summary, gestational age impacted on local gyrification which interacted again with gestational age on neuropsychological performance in adulthood in specific cognitive domains, which have previously been related to these cortical regions.
Discussion
We investigated associations between birth weight, gestational age and gyrification in a large sample of adults, excluding high risk pregnancies and births. We then explored the impact of these associations on neurocognition. We found a positive linear association between gestational age and gyrification bilaterally in the superior frontal cortex, the left supramarginal cortex and in the lingual cortex bilaterally. The association between gyrification clusters and the neuropsychological factors language and working memory/attention was moderated by gestational age. These findings provide an important basis for understanding prenatal influences on brain morphology and their relations to cognitive functions in healthy adults.
We demonstrate positive, linear associations between duration of pregnancy and gyrification in healthy adults. We extracted gyrification values using an absolute mean curvature approach which is positively correlated with increases in total surface area [64]. A methodological strength of this study is the exclusion of participants whose mothers had high-risk pregnancies (e.g., infections, malnutrition, drug abuse, etc.; see above) or pregnancies that are known to be associated with aberrant cortical folding in the fetuses (i.e., multiple pregnancy). Due to this approach, we can conclude that strong associations between rather subtle environmental influences (measured as gestational age) and cortical formation evidently persist in adults even when excluding more severe environmental effects during pregnancy that could have confounded these results. These findings emphasize the formative character from gestational age on brain anatomy which can already be observed in new-borns [87] and shed new light on the importance of preserving maternal health during pregnancy.
Our findings can be interpreted in several ways. First, prenatal cortical growth that is interrupted due to early birth might postnatally not be completed because cortical maturation could potentially only be completed in utero. Second, postnatal compensatory mechanisms of cortical maturation could fail because the harming cause (e.g., maternal stress [15, 88] or smoking [89, 90]) that lead to reduced gestational age still persists in postnatal environment. The third explanation comes from a core assumption of the concept of Developmental Origins of Health and Disease [7, 91, 92]. It posits prenatal mechanisms of permanent fetal programming that are triggered by in utero environmental effects that result in persistent modifications on the epigenome of the differentiating brain cells, which can lead to permanent physiological modifications in the offspring [15]. The consequences of this in utero adaption are twofold: On the one hand, these altered cortical formations are due to the plasticity of the fetal brain which prepares the unborn for its postnatal environment—assuming it is similar to its environment in utero [93]. Thereby, chances of immediate survival after birth and potential reproduction are maximized [94]. On the other hand, these effects can also result in non-adaptive physiology when there is a mismatch between prenatal adaptive brain development and the biological demands that the postnatal environment places on the new-born child. This maladjustment could then lead to vulnerability for later mental disorders.
Our second main result is that gestational age moderates the relationship between localized gyrification and the neuropsychological factors language and working memory/attention. The association between a cluster mostly comprising the left superior frontal cortex and the factor working memory/attention was moderated by gestational age. The involvement of the superior frontal gyrus in working memory has been demonstrated consistently [95]. Additionally, relationships between a gyrification cluster in the left supramarginal gyrus and the factor language as well as the factor working memory/attention were both again moderated by gestational duration. The supramarginal gyrus, as part of Wernicke’s area, has repeatedly been linked to phonological decisions [96, 97], syntax [98] and semantics [99, 100]. Another line of research showed that the supramarginal gyrus is involved in verbal/auditory working memory [101, 102]. Therefore, our results could demonstrate that variations in working memory and language performance are to a certain extent a product of the interactional effect of gestational age and gyrification alterations that are linked to prenatal cortical development. These findings are an important contribution to the identification of neurobiological pathways involved in the association between preterm birth and lower cognitive performance in adults that have been reported in epidemiological studies [4].
Since we found only one direct bivariate association between a gyrification cluster significantly associated with gestational age and one neuropsychological factor but moderated effects it should be pointed out that alterations in gyrification alone might not be sufficient to explain some aspects of poorer neuropsychological outcome. Rather, it is an interplay between variation in gyrification and other neurobiological factors that are influenced by gestational age. This highlights again the importance of determining relationships between prenatal and early-life factors influencing cognitive ability in adulthood.
We found no brain morphological associations with birth weight when we applied a strict statistic (FWE in SPM), although birth weight and gestational age were correlated (r = 0.378; p < 0.001). Gestational age might be a more valid proxy for brain maturation because birth weight is affected by many variables, such as maternal ethnicity, infant gender, maternal smoking and maternal diabetes [103].
Our findings also have implications for psychopathology. Cohort studies have shown that low gestational age is associated with a higher chance to develop a mental disorder in adulthood [104, 105]. Individuals who are at high risk of developing schizophrenia or who will later develop schizophrenia show general cognitive deficits in various domains before the onset of the disorder, including language, processing speed, working memory, executive functioning and intelligence [106, 107]. On a brain morphological level, there are gyrification differences between individuals with higher risk for schizophrenia and control subjects that partly overlap with cortical areas that were associated with gestational age in our study [108, 109]. We suggest that the interaction effect between gestational age and gyrification alterations associated with shortened gestational length may constitute a risk phenotype for mental disorders clinically characterized by reduced cognitive performance.
Limitations and future studies
First, we asked our subjects to state their gestational age, birth weight and their mothers’ pregnancy courses using questionnaires and did not have their birth certificates available. Second, we used both birth weight and gestational age as a summary proxy for the success of prenatal neurodevelopment which is affected by a range of different materno-fetal factors. Future studies should also include patients in their samples to investigate potential interactive effects between gestational duration and pathogenic factors on gyrification as well as cognition.
Conclusion
This study links aberrant local gyrification associated with gestational age to particular neuropsychological domains in healthy adults. We show positive associations between the duration of pregnancy and gyrification alterations in the superior frontal, lingual, and left supramarginal cortex in healthy adults. Additionally, relationships between aberrant gyrification clusters and the factors working memory/attention and language were moderated by gestational age. Our findings demonstrate that effects of prenatal development are permanently present in adult gyrification and have interactional effects on specific cognitive domains. Consequently, our study expands on the importance of maternal health during pregnancy and functions as an impetus to further improve maternal health during pregnancy to preserve cognitive abilities in offspring.
Data availability statement
Research data are not shared.
References
Schlotz W, Phillips DIW (2009) Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun 23(7):905–916
Grove BJ, Lim SJ, Gale CR, Shenkin SD (2017) Birth weight and cognitive ability in adulthood: a systematic review and meta-analysis. Intelligence 61:146–158
Kormos CE, Wilkinson AJ, Davey CJ, Cunningham AJ (2014) Low birth weight and intelligence in adolescence and early adulthood: a meta-analysis. J Public Health 36(2):213–224
Eryigit Madzwamuse S, Baumann N, Jaekel J, Bartmann P, Wolke D (2015) Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J Child Psychol Psychiatry 56(8):857–864
Vohr B (2014) Speech and language outcomes of very preterm infants. Semin Fetal Neonatal Med 19(2):78–83
Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D (2017) Neonatal predictors of cognitive ability in adults born very preterm: a prospective cohort study. Dev Med Child Neurol 59(5):477–483
Gillman MW (2005) Developmental origins of health and disease. N Engl J Med 353(17):1848–1850
Nkansah-Amankra S, Luchok KJ, Hussey JR, Watkins K, Liu X (2010) Effects of maternal stress on low birth weight and preterm birth outcomes across neighborhoods of South Carolina, 2000–2003. Matern Child Health J 14(2):215–226
Khashan A, Everard C, McCowan L, Dekker G, Moss-Morris R, Baker P, Poston L, Walker J, Kenny L (2014) Second-trimester maternal distress increases the risk of small for gestational age. Psychol Med 44(13):2799
Ramos IF, Guardino CM, Mansolf M, Glynn LM, Sandman CA, Hobel CJ, Schetter CD (2019) Pregnancy anxiety predicts shorter gestation in Latina and non-Latina white women: the role of placental corticotrophin-releasing hormone. Psychoneuroendocrinology 99:166–173
Dunkel Schetter C (2011) Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol 62:531–558
Ko T-J, Tsai L-Y, Chu L-C, Yeh S-J, Leung C, Chen C-Y, Chou H-C, Tsao P-N, Chen P-C, Hsieh W-S (2014) Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol 55(1):20–27
Ramakrishnan U (2004) Nutrition and low birth weight: from research to practice. Am J Clin Nutr 79(1):17–21
Basso O, Olsen J, Johansen AMT, Christensen K (1997) Change in social status and risk of low birth weight in Denmark: population based cohort study. BMJ 315(7121):1498–1502
Franke K, Van den Bergh BRH, de Rooij SR, Kroegel N, Nathanielsz PW, Rakers F, Roseboom TJ, Witte OW, Schwab M (2020) Effects of maternal stress and nutrient restriction during gestation on offspring neuroanatomy in humans. Neurosci Biobeh Reviews 117:5–25
Engelhardt E, Inder TE, Alexopoulos D, Dierker DL, Hill J, Van Essen D, Neil JJ (2015) Regional impairments of cortical folding in premature infants. Ann Neurol 77(1):154–162
Hedderich DM, Bäuml JG, Berndt MT, Menegaux A, Scheef L, Daamen M, Zimmer C, Bartmann P, Boecker H, Wolke D et al (2019) Aberrant gyrification contributes to the link between gestational age and adult IQ after premature birth. Brain 142(5):1255–1269
Papini C, Palaniyappan L, Kroll J, Froudist-Walsh S, Murray RM, Nosarti C (2020) Altered cortical gyrification in adults who were born very preterm and its associations with cognition and mental health. Biol Psychiatry 5(7):640–650
Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K (1995) The ontogeny of human gyrification. Cereb Cortex 5(1):56–63
Cao B, Mwangi B, Passos IC, Wu M-J, Keser Z, Zunta-Soares GB, Xu D, Hasan KM, Soares JC (2017) Lifespan gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci Rep 7(1):511
Sydnor VJ, Larsen B, Bassett DS, Alexander-Bloch A, Fair DA, Liston C, Mackey AP, Milham MP, Pines A, Roalf DR et al (2021) Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron 109(18):2820–2846
Kersbergen KJ, Leroy F, Išgum I, Groenendaal F, de Vries LS, Claessens NHP, van Haastert IC, Moeskops P, Fischer C, Mangin J-F et al (2016) Relation between clinical risk factors, early cortical changes, and neurodevelopmental outcome in preterm infants. Neuroimage 142:301–310
Gregory MD, Kippenhan JS, Dickinson D, Carrasco J, Mattay VS, Weinberger DR, Berman KF (2016) Regional variations in brain gyrification are associated with general cognitive ability in humans. Curr Biol 26(10):1301–1305
Chung YS, Hyatt CJ, Stevens MC (2017) Adolescent maturation of the relationship between cortical gyrification and cognitive ability. Neuroimage 158:319–331
Zilles K, Palomero-Gallagher N, Amunts K (2013) Development of cortical folding during evolution and ontogeny. Trends Neurosci 36(5):275–284
Dubois J, Lefèvre J, Angleys H, Leroy F, Fischer C, Lebenberg J, Dehaene-Lambertz G, Borradori-Tolsa C, Lazeyras F, Hertz-Pannier L et al (2019) The dynamics of cortical folding waves and prematurity-related deviations revealed by spatial and spectral analysis of gyrification. Neuroimage 185:934–946
Clouchoux C, Kudelski D, Gholipour A, Warfield SK, Viseur S, Bouyssi-Kobar M, Mari J-L, Evans AC, Du Plessis AJ, Limperopoulos C (2012) Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct 217(1):127–139
Haukvik UK, Schaer M, Nesvåg R, McNeil T, Hartberg CB, Jönsson EG, Eliez S, Agartz I (2012) Cortical folding in Broca’s area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol Med 42(6):1329–1337
Hendrickson TJ, Mueller BA, Sowell ER, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lim KO, Riley EP et al (2017) Cortical gyrification is abnormal in children with prenatal alcohol exposure. Neuroimage 15:391–400
Kircher T, Wöhr M, Nenadic I, Schwarting R, Schratt G, Alferink J, Culmsee C, Garn H, Hahn T, Müller-Myhsok B et al (2018) Neurobiology of the major psychoses: a translational perspective on brain structure and function—the FOR2107 consortium. Eur Arch Psychiatry Clin Neurosci 269(8):949–962
Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M (1997) SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I
Lehrl S (1995) Mehrfachwahl-Wortschatz-intelligenztest: MWT-B. Hogrefe, Göttingen
Nelson HE, Willison J (1991) National adult reading test (NART). Nfer-Nelson Windsor
Dammann O, Drescher J, Veelken N (2003) Maternal fever at birth and non-verbal intelligence at age 9 years in preterm infants. Dev Med Child Neurol 45(3):148–151
Goldenberg RL, Culhane JF, Johnson DC (2005) Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol 32(3):523–559
Haley DW, Handmaker NS, Lowe J (2006) Infant stress reactivity and prenatal alcohol exposure. Alcoholism 30(12):2055–2064
Sayal K, Heron J, Golding J, Emond A (2007) Prenatal alcohol exposure and gender differences in childhood mental health problems: a longitudinal population-based study. Pediatrics 119(2):e426
Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN (2007) Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics 119(3):e733
Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R (2007) Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics 119(2):e348
Bennett DS, Bendersky M, Lewis M (2008) Children’s cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol 44(4):919
Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min MO, Kirchner HL (2004) Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA 291(20):2448–2456
Goldschmidt L, Richardson GA, Willford J, Day NL (2008) Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry 47(3):254–263
Huizink AC, Mulder EJH (2006) Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev 30(1):24–41
Georgieff MK (2007) Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 85(2):614S-620S
Gale CR, Robinson SM, Godfrey KM, Law CM, Schlotz W, O’Callaghan FJ (2008) Oily fish intake during pregnancy—association with lower hyperactivity but not with higher full-scale IQ in offspring. J Child Psychol Psychiatry 49(10):1061–1068
Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J (2007) Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. The Lancet 369(9561):578–585
Parsons AG, Zhou SJ, Spurrier NJ, Makrides M (2008) Effect of iron supplementation during pregnancy on the behaviour of children at early school age: long-term follow-up of a randomised controlled trial. Br J Nutr 99(5):1133–1139
Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M (2006) Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr 83(5):1112–1117
Schlotz W, Jones A, Phillips DIW, Gale CR, Robinson SM, Godfrey KM (2010) Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry 51(5):594–602
Esteban FJ, Padilla N, Sanz-Cortés M, de Miras JR, Bargalló N, Villoslada P, Gratacós E (2010) Fractal-dimension analysis detects cerebral changes in preterm infants with and without intrauterine growth restriction. Neuroimage 53(4):1225–1232
Quezada S, Castillo-Melendez M, Walker DW, Tolcos M (2018) Development of the cerebral cortex and the effect of the intrauterine environment. J Physiol 596(23):5665–5674
Ingram Cooke RW (2010) Does neonatal and infant neurodevelopmental morbidity of multiples and singletons differ? Semin Fetal Neonatal Med 15(6):362–366
Zornberg GL, Buka SL, Tsuang MT (2000) Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry 157(2):196–202
Goldstein JM, Seidman LJ, Buka SL, Horton NJ, Donatelli JL, Rieder RO, Tsuang MT (2000) Impact of genetic vulnerability and hypoxia on overall intelligence by age 7 in offspring at high risk for schizophrenia compared with affective psychoses. Schizophr Bull 26(2):323–334
Wortinger LA, Engen K, Barth C, Andreassen OA, Nordbø Jørgensen K, Agartz I (2020) Asphyxia at birth affects brain structure in patients on the schizophrenia-bipolar disorder spectrum and healthy participants. Psychol Med 52(6):1050–1059
Wortinger LA, Engen K, Barth C, Lonning V, Jørgensen KN, Andreassen OA, Haukvik UK, Vaskinn A, Ueland T, Agartz I (2020) Obstetric complications and intelligence in patients on the schizophrenia-bipolar spectrum and healthy participants. Psychol Med 50(11):1914–1922
Dbstet A (1977) WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Acta Obstet Gynecol Scand 56(3):247–253
Vogelbacher C, Möbius TWD, Sommer J, Schuster V, Dannlowski U, Kircher T, Dempfle A, Jansen A, Bopp MHA (2018) The Marburg-Münster Affective Disorders Cohort Study (MACS): a quality assurance protocol for MR neuroimaging data. Neuroimage 172:450–460
Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE (2011) Statistical parametric mapping: the analysis of functional brain images. Elsevier
Dahnke R, Yotter RA, Gaser C (2013) Cortical thickness and central surface estimation. Neuroimage 65:336–348
Yotter RA, Dahnke R, Thompson PM, Gaser C (2011) Topological correction of brain surface meshes using spherical harmonics. Hum Brain Mapp 32(7):1109–1124
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113
Yotter RA, Thompson PM, Gaser C (2011) Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J Neuroimaging 21(2):e134-147
Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C (2006) A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage 29(4):1224–1230
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BTJN (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44(1):83–98
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92:381–397
O’Gorman TW (2005) The performance of randomization tests that use permutations of independent variables. Commun Stat 34(4):895–908
Flandin G, Friston KJ (2019) Analysis of family-wise error rates in statistical parametric mapping using random field theory. Hum Brain Mapp 40(7):2052–2054
Nichols T, Hayasaka S (2003) Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res 12(5):419–446
Aschenbrenner S, Tucha O, Lange KW (2000) Regensburger wortflüssigkeits-test: RWT. Hogrefe, Verlag für Psychologie
Reitan RM (1958) Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 8(3):271–276
Wechsler D: WAiS-iii: Psychological Corporation San Antonio, TX; 1997.
Brickenkamp R, Schmidt-Atzert L, Liepmann D (2010) Test d2—revision aufmerksamkeits und konzentrationstest (Manual). Hogrefe, Göttingen
Helmstaedter C, Durwen H (1990) VLMT: Verbaler Lern-und Merkfähigkeitstest: Ein praktikables und differenziertes Instrumentarium zur Prüfung der verbalen Gedächtnisleistungen. Schweizer Archiv für Neurologie, Neurochirurgie und Psychiatrie
Corsi P (1972) Memory and the medial temporal region of the brain. Unpublished doctoral dissertation), McGill University, Montreal
IBM (2016) IBM SPSS statistics for Mac, version 24.0. IBM Corp, Armonk
Cattell RB (1966) The scree test for the number of factors. Multivar Behav Res 1(2):245–276
Nosarti C, Nam KW, Walshe M, Murray RM, Cuddy M, Rifkin L, Allin MPG (2014) Preterm birth and structural brain alterations in early adulthood. Neuroimage 6:180–191
Ganella EP, Burnett A, Cheong J, Thompson D, Roberts G, Wood S, Lee K, Duff J, Anderson PJ, Pantelis C et al (2015) Abnormalities in orbitofrontal cortex gyrification and mental health outcomes in adolescents born extremely preterm and/or at an extremely low birth weight. Hum Brain Mapp 36(3):1138–1150
Kelly CE, Thompson DK, Cheong JLY, Chen J, Olsen JE, Eeles AL, Walsh JM, Seal ML, Anderson PJ, Doyle LW et al (2019) Brain structure and neurological and behavioural functioning in infants born preterm. Dev Med Child Neurol 61(7):820–831
Martinussen M, Flanders DW, Fischl B, Busa E, Løhaugen GC, Skranes J, Vangberg TR, Brubakk A-M, Haraldseth O, Dale AM (2009) Segmental brain volumes and cognitive and perceptual correlates in 15-year-old adolescents with low birth weight. J Pediatr 155(6):848-853.e841
Hayes AF (2017) Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Guilford publications, Berlin
Duff K, Patton D, Schoenberg MR, Mold J, Scott JG, Adams RL (2003) Age-and education-corrected independent normative data for the RBANS in a community dwelling elderly sample. Clin Neuropsychol 17(3):351–366
Davidson R, MacKinnon JG (1993) Estimation and inference in econometrics. OUP Catalogue, Berlin
Long JS, Ervin LH (2000) Using heteroscedasticity consistent standard errors in the linear regression model. Am Stat 54(3):217–224
Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR (2006) Increased temporal lobe gyrification in preterm children. Neuropsychologia 44(3):445–453
van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S et al (2017) Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev 117:26–64
Abbott LC, Winzer-Serhan UH (2012) Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol 42(4):279–303
Ekblad M, Korkeila J, Parkkola R, Lapinleimu H, Haataja L, Lehtonen L (2010) Maternal smoking during pregnancy and regional brain volumes in preterm infants. J Pediatr 156(2):185-190.e181
Gluckman PD, Hanson MA, Buklijas T (2010) A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis 1(1):6–18
Davis EP, Glynn LM, Waffarn F, Sandman CA (2011) Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry 52(2):119–129
Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM et al (2004) Developmental plasticity and human health. Nature 430(6998):419–421
Hanson MA, Gluckman PD (2014) Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94(4):1027–1076
Wager TD, Smith EE (2003) Neuroimaging studies of working memory. Cogn Affect Behav Neurosci 3(4):255–274
Sliwinska MW, Khadilkar M, Campbell-Ratcliffe J, Quevenco F, Devlin J (2012) Early and sustained supramarginal gyrus contributions to phonological processing. Front Psychol. https://doi.org/10.3389/fpsyg.2012.00161
Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR (2010) Phonological decisions require both the left and right supramarginal gyri. Proc Natl Acad Sci 107(38):16494
Price CJ (2010) The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191(1):62–88
Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E (2005) Some neurophysiological constraints on models of word naming. Neuroimage 27(3):677–693
Dębska A, Chyl K, Dzięgiel G, Kacprzak A, Łuniewska M, Plewko J, Marchewka A, Grabowska A, Jednoróg K (2019) Reading and spelling skills are differentially related to phonological processing: behavioral and fMRI study. Dev Cogn Neurosci 39:100683
Deschamps I, Baum SR, Gracco VL (2014) On the role of the supramarginal gyrus in phonological processing and verbal working memory: evidence from rTMS studies. Neuropsychologia 53:39–46
Vines BW, Schnider NM, Schlaug G (2006) Testing for causality with transcranial direct current stimulation: pitch memory and the left supramarginal gyrus. NeuroReport 17(10):1047–1050
Amini SB, Catalano PM, Hirsch V, Mann LI (1994) An analysis of birth weight by gestational age using a computerized perinatal data base, 1975–1992. Obstet Gynecol 83(3):342–352
Xie S, Heuvelman H, Magnusson C, Rai D, Lyall K, Newschaffer CJ, Dalman C, Lee BK, Abel K (2017) Prevalence of autism spectrum disorders with and without intellectual disability by gestational age at birth in the stockholm youth cohort: a register linkage study. Paediatr Perinat Epidemiol 31(6):586–594
Burnett A, Anderson P, Cheong J, Doyle L, Davey C, Wood S (2011) Prevalence of psychiatric diagnoses in preterm and full-term children, adolescents and young adults: a meta-analysis. Psychol Med 41(12):2463
Mollon J, Reichenberg A (2018) Cognitive development prior to onset of psychosis. Psychol Med 48(3):392
Bora E, Murray RM (2013) Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull 40(4):744–755
Sasabayashi D, Takayanagi Y, Takahashi T, Koike S, Yamasue H, Katagiri N, Sakuma A, Obara C, Nakamura M, Furuichi A et al (2017) Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol Psychiat 82(10):737–745
Hou J, Schmitt S, Meller T, Falkenberg I, Chen J, Wang J, Zhao X, Shi J, Nenadić I (2020) Cortical complexity in people at ultra-high-risk for psychosis moderated by childhood trauma. Front Psychiatry. https://doi.org/10.3389/fpsyt.2020.594466
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Acknowledgements
This work is part of the German multicentre consortium “Neurobiology of Affective Disorders. A translational perspective on brain structure and function”, funded by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG; Forschungsgruppe/Research Unit FOR2107). Please visit https://for2107.de/acknowledgements/?lang=en for full FOR2107 acknowledgments.
Funding
Open Access funding enabled and organized by Projekt DEAL. Principal investigators (PIs) with respective areas of responsibility in the FOR2107 consortium are: Work Package WP1, FOR2107/MACS cohort and brainimaging: Tilo Kircher (speaker FOR2107; DFG grant numbers KI 588/14-1, KI 588/14-2), Udo Dannlowski (co-speaker FOR2107; DA 1151/5-1, DA 1151/5-2), Axel Krug (KR 3822/5-1, KR 3822/7-2), Igor Nenadić (NE 2254/1-2), Carsten Konrad (KO 4291/3-1). WP2, animal phenotyping: Markus Wöhr (WO 1732/4-1, WO 1732/4-2), Rainer Schwarting (SCHW 559/14-1, SCHW 559/14-2). WP3, miRNA: Gerhard Schratt (SCHR 1136/3-1, 1136/3-2). WP4, immunology, mitochondriae: Judith Alferink (AL 1145/5-2), Carsten Culmsee (CU 43/9-1, CU 43/9-2), Holger Garn (GA 545/5-1, GA 545/7-2). WP5, genetics: Marcella Rietschel (RI 908/11-1, RI 908/11-2), Markus Nöthen (NO 246/10-1, NO 246/10-2), Stephanie Witt (WI 3439/3-1, WI 3439/3-2). WP6, multi-method data analytics: Andreas Jansen (JA 1890/7-1, JA 1890/7-2), Tim Hahn (HA 7070/2-2), Bertram Müller-Myhsok (MU1315/8-2), Astrid Dempfle (DE 1614/3-1, DE 1614/3-2). CP1, biobank: Petra Pfefferle (PF 784/1-1, PF 784/1-2), Harald Renz (RE 737/20-1, 737/20-2). CP2, administration. Tilo Kircher (KI 588/15-1, KI 588/17-1), Udo Dannlowski (DA 1151/6-1), Carsten Konrad (KO 4291/4-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Biomedical financial interests or potential conflicts of interest: Tilo Kircher received unrestricted educational grants from Servier, Janssen, Recordati, Aristo, Otsuka, neuraxpharm. All other authors have nothing to disclose.
Data access and responsibility
All PIs take responsibility for the integrity of the respective study data and their components. All authors and coauthors had full access to all study data.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmitt, S., Ringwald, K.G., Meller, T. et al. Associations of gestational age with gyrification and neurocognition in healthy adults. Eur Arch Psychiatry Clin Neurosci 273, 467–479 (2023). https://doi.org/10.1007/s00406-022-01454-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01454-0