Abstract
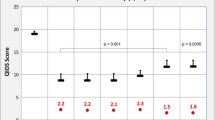
Results of a preclinical study suggested that the anticonvulsant drug ethosuximide may elicit ketamine-like rapid-acting antidepressant actions. We evaluated the antidepressant efficacy of ethosuximide versus placebo in non-medicated adult patients with major depressive disorder (MDD). This randomized, double-blind, placebo-controlled trial included patients at three mental health centers in China. Eighty eligible adults (aged 18–65 years) met the DSM-5 criteria for MDD. Patients in the acute single study received three doses (500, 1000, or 1500 mg) of ethosuximide or placebo. Patients in the repeated study received ethosuximide (1500 mg/day) or placebo for 2 weeks. The Hamilton Depression Rating Scale (HAM-D), the Montgomery–Åsberg Depression Rating Scale (MADRS), and the Hamilton Anxiety Rating Scale were used to assess antidepressant and antianxiety responses to ethosuximide. No significant reductions in depression and anxiety rating scale scores were observed after a single oral administration of ethosuximide, in comparison with placebo. Furthermore, patients receiving ethosuximide for 2 weeks did not show reductions in depression and anxiety rating scale scores. There were no serious adverse events. Responses to the study’s primary and secondary outcome measures, the clinician-rated HAM-D and MADRS, showed no change from baseline to the end of treatment, with either ethosuximide or placebo. These results suggest that ethosuximide does not produce ketamine-like robust antidepressant actions in adult patients with MDD.



Similar content being viewed by others
References
Trivedi MH (2016) Dose response for SSRIs. Am J Psychiatry 173(2):105–106. https://doi.org/10.1176/appi.ajp.2015.15121535
Hashimoto K (2016) Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Exp Opinion Ther Targets 20(11):1389–1392. https://doi.org/10.1080/14728222.2016.1238899
Murrough JW, Abdallah CG, Mathew SJ (2017) Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 16(7):472–486. https://doi.org/10.1038/nrd.2017.16
Duman RS (2018) Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Research. https://doi.org/10.12688/f1000research.14344.1
Hashimoto K (2019) Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci 73(10):613–627. https://doi.org/10.1111/pcn.12902
Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS (2019) Ketamine: a paradigm shift for depression research and treatment. Neuron 101(5):774–778. https://doi.org/10.1016/j.neuron.2019.02.005
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354. https://doi.org/10.1016/S0006-3223(99)00230-9
Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8):856–864. https://doi.org/10.1001/archpsyc.63.8.856
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170(10):1134–1142. https://doi.org/10.1176/appi.ajp.2013.13030392
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74(4):250–256. https://doi.org/10.1016/j.biopsych.2012.06.022
Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, APA Council of Research Task Force on Novel Biomarkers and Treatments (2015) Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172(10):950–966. https://doi.org/10.1176/appi.ajp.2015.15040465
Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU (2016) Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46(7):1459–1472. https://doi.org/10.1017/S0033291716000064
Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173(8):816–826. https://doi.org/10.1176/appi.ajp.2016.16010037
Su TP, Chen MH, Li CT, Lin WC, Hong CJ, Gueorguieva R, Tu PC, Bai YM, Cheng CM, Krystal JH (2017) Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology 42(13):2482–2492. https://doi.org/10.1038/npp.2017.94
Chen MH, Lin WC, Tu PC, Li CT, Bai YM, Tsai SJ, Su TP (2019) Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: a double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J Affect Disord 259:15–20. https://doi.org/10.1016/j.jad.2019.08.022
Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI (2019) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. https://doi.org/10.1038/s41380-018-0256-5
Wilkinson ST, Sanacora G (2017) Considerations on the off-label use of ketamine as a treatment for mood disorders. JAMA 318(9):793–794. https://doi.org/10.1001/jama.2017.10697
Reardon S (2018) ‘Party drug’ turned antidepressant approaches approval. Nat Rev Drug Discov 17(11):773–775. https://doi.org/10.1038/nrd.2018.187
Hashimoto K (2016) Detrimental side effects of repeated ketamine infusions in the brain. Am J Psychiatry 173(10):1044–1045. https://doi.org/10.1176/appi.ajp.2016.16040411
Hashimoto K (2016) R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med 46(11):2449–2451. https://doi.org/10.1017/S0033291716000969
Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments (2017) A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74(4):399–405. https://doi.org/10.1001/jamapsychiatry.2017.0080
Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R (2017) Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight. Lancet Psychiatry 4(5):419–426. https://doi.org/10.1016/S2215-0366(17)30102-5
Short B, Fong J, Galvez V, Shelker W, Loo CK (2018) Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5(1):65–78. https://doi.org/10.1016/S2215-0366(17)30272-9
Zhang K, Hashimoto K (2019) An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Rev Neurother 19(1):83–92. https://doi.org/10.1080/14737175.2019.1554434
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554(7692):317–322. https://doi.org/10.1038/nature25509
Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, Clark PO, Capparelli EV, Adamson PC, Childhood Absence Epilepsy Study Group (2010) Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med 362:790–799. https://doi.org/10.1056/NEJMoa0902014
Brigo F, Igwe SC, Lattanzi S (2019) Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescent. Cochrane Database Syst Rev 2:CD003030. https://doi.org/10.1002/14651858.CD003032.pub4
Gören MZ, Onat F (2007) Ethosuximide: from bench to bedside. CNS Drug Rev 13(2):224–239. https://doi.org/10.1111/j.1527-3458.2007.00009.x
Fischman MW, Foltin RW (1991) Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict 86(12):1563–1570. https://doi.org/10.1111/j.1360-0443.1991.tb01749.x
Iovieno N, Papakostas GI (2012) Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry 73(10):1300–1306. https://doi.org/10.4088/JCP.11r07485
Kerckhove N, Pereira B, Soriot-Thomas S, Alchaar H, Deleens R, Hieng VS, Serra E, Lanteri-Minet M, Arcagni P, Picard P, Lefebvre-Kuntz D, Maindet C, Mick G, Balp L, Lucas C, Creach C, Letellier M, Martinez V, Navez M, Delbrouck D, Kuhn E, Piquet E, Bozzolo E, Brosse C, Lietar B, Marcaillou F, Hamdani A, Leroux-Bromberg N, Perier Y, Vergne-Salle P, Gov C, Delage N, Gillet D, Romettino S, Richard D, Mallet C, Bernard L, Lambert C, Dubray C, Duale C, Eschalier A (2018) Efficacy and safety of a T-type calcium channel blocker in patients with neuropathic pain: a proof-of-concept, randomized, double-blind and controlled trial. Eur J Pain 22(7):1321–1330. https://doi.org/10.1002/ejp.1221
Sarkisova K, van Luijtelaar G (2011) The WAG/Rij strain: a genetic animal model of absence epilepsy with comorbidity of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35(4):854–876. https://doi.org/10.1016/j.pnpbp.2010.11.010
Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2018) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83(1):18–28. https://doi.org/10.1016/j.biopsych.2017.05.016
Leo A, Citrao R, Tallarico M, Iannone M, Fedosova E, Nesci V, De Sarro G, Sarkisova K, Russo E (2019) Cognitive impairment in the WAG/Rij rat absence model is secondary to absence seizures and depressive-like behavior. Prog Neuro-Psychopharmacol Biol Psychiatry 2019:109652. https://doi.org/10.1016/j.pnpbp.2019.109652
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632
Zhang K, Ma M, Dong C, Hashimoto K (2018) Role of inflammatory bone markers in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol 21(11):1025–1030. https://doi.org/10.1093/ijnp/pyy065
Tian Z, Dong C, Zhang K, Chang L, Hashimoto K (2018) Lack of antidepressant effects of low-voltage-sensitive T-type calcium channel blocker ethosuximide in a chronic social defeat stress model: comparison with (R)-ketamine. Int J Neuropsychopharmacol 21(11):1031–1036. https://doi.org/10.1093/ijnp/pyy072
Jiang J, Wang Z, Dong Y, Yang Y, Ng CH, Ma S, Xu Y, Hu H, Hu S (2019) A statistical analysis plan for a randomized clinical trial to evaluate the efficacy and safety of ethosuximide in patients with treatment-resistant depression. Medicine (Baltimore) 98(31):e16674. https://doi.org/10.1097/MD.0000000000016674
Acknowledgements
We thank all the patients who volunteered to participate in the study. This study was supported by the National Nature Science Foundation of China (81801341), the Medical and Public Health Technology Research Projects of Wuxi Technology Bureau (CSE31N1723), the Wuxi Municipal Health and Family Planning Commission research project (MS201704), and AMED, Japan (to K.H., JP19dm0107119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Hashimoto is an inventor on a filed patent application on “Application of R-ketamine and salt thereof as pharmaceuticals” by Chiba University. Dr. Hashimoto has received research support from Otsuka, Sumitomo-Dainippon, and Taisho. The other authors report no financial relationships with commercial interests.
Rights and permissions
About this article
Cite this article
Zhang, K., Jia, G., Xia, L. et al. Efficacy of anticonvulsant ethosuximide for major depressive disorder: a randomized, placebo-control clinical trial. Eur Arch Psychiatry Clin Neurosci 271, 487–493 (2021). https://doi.org/10.1007/s00406-020-01103-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-020-01103-4