Abstract
Background
Functional imaging studies in healthy individuals revealed an association between 5-HTTLPR genotype and neuronal activity in the amygdala. The aim of this study was firstly to investigate a possible overall impact of the 5-HTTLPR on amygdala volume in patients with bipolar disorder and healthy individuals and secondly to test a diagnosis specific influence of the 5-HTTLPR on amygdala volume.
Methods
We performed a region of interest analysis of amygdala volume in 37 patients with bipolar I disorder and 37 healthy control subjects. The 5-HTTLPR genotype of each proband was determined and the subjects were separated according to 5-HTTLPR genotype and for statistical analyses the groups SS and SL were combined and compared with the group LL.
Results
This study shows that carriers of the short allele (SL or SS) of the 5-HTTLPR polymorphism exhibit a relatively increased volume of the right amygdala compared to homozygous L-allele carriers irrespective of diagnosis status. However, further analyses with the factors genotype and diagnosis were not able to reproduce this result.
Conclusions
The present findings are consistent with the view that the 5-HTTLPR polymorphism might modulate neuronal size or number in the amygdala. It would be worthwhile investigating the relationship between serotonin transporter function and amygdala function and volume in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Objectives of the study
The amygdala is a key structure for the modulation of emotional behaviour. Several lines of evidence suggest that the amygdala is structurally and functionally altered in patients with bipolar disorder [12, 14]. In adult patients with bipolar disorder three studies observed an enlargement of the amygdala compared to healthy subjects [14]. In contrast to this, studies investigating adolescent patients reported a decreased amygdala volume [1]. A meta-analysis of volumes of regional brain structures in bipolar disorder, however, could not observe any significant change of amygdala volume [9]. Another recent systematic review and meta-analysis of amygdala volume in adolescent and adult patients with bipolar disorder included 14 MRI studies [13]. This study revealed a bilaterally reduced amygdala volume in the overall sample of patients with bipolar disorder, but the results of the individual studies and the pediatric and adult subsamples were heterogeneous. An additional meta-regression analysis revealed a positive correlation between mean age and amygdala volume in patients with bipolar disorder [13]. Therefore, the authors concluded that amygdala volume is decreased at the beginning of the disorder and increases with illness duration. This hypothesis is supported by Chen and coworkers [3] who reported a positive correlation of age and amygdala volume in patients with bipolar disorder in contrast to a negative correlation in healthy control subjects.
Functional imaging studies with depressed patients suffering from bipolar disorder exhibited a disturbed cortical-limbic network with increased metabolism in amygdala and other subcortical structures [7]. Another study observed a reduced dorsolateral prefrontal cortical activation corresponding with increased amygdala activation in a facial affect discrimination task in patients with bipolar disorder [19]. Taken together, these results suggest an involvement of the amygdala in the pathophysiology of bipolar disorder.
The neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) is an essential modulator of emotional behaviour and an abnormal function of 5-HT is found in mood disorders [4]. The serotonin transporter plays an important role in regulating 5-HT levels in the brain by transporting 5-HT from the synaptic cleft to the pre-synaptic neuron. A recent study reported a 26% lower serotonin transporter binding potential in the amygdalae of depressed medication-free patients with bipolar disorder [10]. A polymorphism (5-HT transporter-linked polymorphic region; 5-HTTLPR) in the transcriptional control region upstream of the coding sequence of the serotonin transporter gene (SLC6A4) results in short (S)- or long (L)-alleles [8]. The S-allele is associated with significant lower serotonin transporter binding and mRNA expression [4].
Functional imaging studies in healthy individuals revealed an association between 5-HTTLPR genotype and neuronal activity in the amygdala. Carriers of the S-allele showed reduced serotonin expression and an increased fear and anxiety-related behaviour [8]. Furthermore, in functional magnetic resonance imaging it could be shown that carriers of the S-allele exhibited greater amygdala neuronal activity in response to fearful stimuli [5] or aversive pictures [6] compared to individuals with the homozygous L genotype. Pezawas and colleagues observed an uncoupling of a cingulate-amygdala feedback circuit in healthy carriers of the S-allele. Moreover, in this study a structural analysis using optimized voxel-based morphometry showed a significantly reduced gray matter volume of the right and left amygdala in S-allele carriers [11]. Taken together, these studies suggest that the 5-HTTLPR genotype might modulate serotonergic function and, moreover, amygdala function as well as structure in healthy individuals.
We hypothesized that the 5-HTTLPR genotype might impact the amygdala volume generally in patients with bipolar disorder as well as in healthy individuals. In the above mentioned studies on amygdala volume in bipolar disorder the influence of the 5-HTTLPR genotype were not considered. The aim of this study was to clarify the role of the 5-HTTLPR genotype on amygdala volume in patients with bipolar I disorder and healthy control subjects. Firstly we tested the overall effect of the 5-HTTLPR genotype on amygdala volume and secondly we looked for a diagnosis specific influence.
Materials and Methods
Subjects
Thirty-seven euthymic patients with bipolar I disorder and 37 healthy control subjects participated in the study (Table 1). Written informed consent was obtained from all subjects and the study was approved by the local ethical committee. Subjects were recruited from the outpatient unit of the Department of Psychiatry and Psychotherapy of the Saarland University Hospital. The diagnosis of bipolar I disorder and the condition as healthy subjects was confirmed by using the German version of the Structural Clinical Interview for DSM-IV [17]. Other axis-I disorders in particular alcohol dependence and medical disorders were excluded. All bipolar disorder patients were receiving stable medication at the time of the study. The healthy control subjects exhibited no past or present psychiatric, neurological or medical disorder and had no positive family history of psychiatric disorders. They were recruited from the general population via advertisement in newspapers.
Imaging
MRI scanning was performed on a 1.5 Tesla Magnetom (Siemens, Erlangen) and a T1-weighted, MPRAGE sequence of 176 consecutive slices with a voxel size of 1 mm3 was acquired. One rater blind to diagnosis and genotype (PM) determined amygdala volume by direct manual tracing of the boundaries as previously described by Brambilla and colleagues [2]. The intra-rater intra-class correlation coefficients (ICC), established by double tracing of 14 scans, were ICC = 0.980 for the right and ICC = 0.978 for the left amygdala. Relative volume was computed by dividing amygdala volume by total gray matter volume.
Genotyping
Genotyping was accomplished essentially as described by Lesch and collaborators [8]. In brief, genomic DNA was extracted from EDTA blood by standard procedures. 5-HTTLPR (SLC6A4)-specific primers (forward, 5′-TAGAGGGACTGAGCTGGACAACCAC-3′; reverse, 5′-GGTGTTGCCGCTCTGAATGC-3′) were used for polymerase chain reaction (PCR). The thermocycler protocol involved an initial denaturation cycle (4 min, 94°C), 35 cycles of denaturation (30 s, 94°C), annealing (30 s, 61.4°C), and extension (30 s, 72°C), followed by a final extension step (7 min, 72°C), and terminating at 10°C. This procedure resulted in fragments of 585 (short, S) and 628 (long, L) bp in length.
Fragments were amplified by PCR in a final volume of 50 µl containing 100 ng genomic DNA, 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 0.025 % Tween 20, 0.025 % BSA, 10 pmol of each primer (MWG Biotech), 0.2 mM of each dNTP (MBI Fermentas), 7.5 mM MgCl2, 5 % DMSO and 2 unit of Taq Polymerase (Eurogentec, Seraing, Belgium). For analysis, 10 µl of PCR product were visualized on 1.5 % agarose gels stained with ethidium bromide.
Statistics
Statistical analysis was performed using SPSS 14. All tests were two-tailed. Significance level was α = 0.05. The subjects were separated according to 5-HTTLPR genotype and for statistical analyses the groups SS and SL were combined and compared with the group LL. With this approach we follow several previous studies which combined all individuals with at least one S-allele in one group [5, 6, 11]. The groups divided by genotype (Table 1) or by genotype and diagnosis (Table 2) did not show significant differences regarding age, education or handedness. However, the female fraction in the group LL was disproportionately high (Table 1), in particular in the control group (Table 2). Therefore, we adjusted the main analyses for gender. Additionally, we adjusted for age considering the negative correlation between brain volume and age and the hypothesized positive correlation between age and amygdala volume in bipolar disorder. Multivariate analyses of covariance (MANCOVA) with 5-HTTLPR polymorphism and gender as independent factors, age as covariate and right and left amygdala volume as dependent variables were calculated (Table 3). Additionally, the factor diagnosis was entered into the model to assess whether interactions between genotype and diagnosis could be found (Table 4).
Results
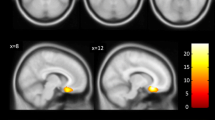
The multivariate analyses with independent factors 5-HTTLPR polymorphism and gender and covariate age revealed a significant effect for 5-HTTLPR genotype on absolute (df = 2, 68, F = 3.9, P < 0.05) and relative (df = 2, 68, F = 3.5, P < 0.05) amygdala volume (Table 3). Following univariate tests showed that the absolute (+12%, df = 1, 69, F = 5.9, P < 0.05) and relative volume of the right amygdala (+12%, df = 1, 69, F = 5.6, P < 0.05) were increased in S-allele carriers compared to individuals with the homozygous L-genotype (Table 3; Fig. 1). Absolute and relative volumes of the right amygdala were increased in S-allele carriers for both groups investigated in this study (healthy controls: absolute volume +11%, relative volume +9.4%; patients with bipolar disorder: absolute volume +10%, relative volume +11.4%). The additional MANCOVA with the independent factors 5-HTTLPR-polymorphism, diagnosis, gender and covariate age was only able to show a statistical trend for the multivariate analysis of the factor 5-HTTLPR on absolute amygdala volumes, though. With respect to the factor diagnosis or the interaction between genotype and diagnosis no significant effect was found (Table 4).
Discussion
In this study we investigated the influence of the 5-HTTLPR polymorphism on amygdala volume in healthy individuals and patients with bipolar disorder. The analyses revealed increased absolute and relative volumes of the right amygdala in all S-allele carriers of the 5-HTTLPR polymorphism. These findings indicate that the 5-HTTLPR genotype has an impact on amygdala volume both in patients with bipolar disorder and healthy control subjects. Further analyses with the additional factor diagnosis were unable to show an effect of genotype or diagnosis on absolute or relative amygdala volume. Even though no interaction between genotype and diagnosis was found, we were not able to show that the increased amygdala volume depends on the genotype only. However, as both healthy individuals and patients with bipolar disorder displayed an increased amygdala volume in the first analysis, we hypothesize a potential general influence of the 5-HTTLPR polymorphism on amygdala volume.
The statistical not significant findings of the second analyses might result from the relatively small sample size of 74 subjects. Nevertheless, we interpret these data as an indication for a possible interaction between 5-HTTLPR-genotype and amygdala volume. Further analyses with a larger sample size might be able to verify these first results.
An increased volume of the amygdala derived from MRI could indicate an increased number of neurons in this brain region. It has been discussed that variations in MRI measurements potentially reflect alterations of neuronal and non-neuronal tissue compartments, physiological alterations in brain tissue or changes in other chemical constituents [16]. However, a recent study using post mortem tissue of the pulvinar from patients with major depressive disorder, bipolar disorder, schizophrenia and healthy controls reported a higher number and volume of pulvinar neurons in subjects with the homozygous short 5-HTTLPR genotype [18]. As both the pulvinar and amygdala contain high levels of serotonin transporter and are connected with each other [18] the possible effects of 5-HTTLPR genotype on neurons might be comparable. We hypothesize, therefore, that the increased amygdala volume in S-allele carriers may reflect a higher number of neurons or an increased volume of neurons in the right amygdala. As we found this relatively increased volume both in healthy control subjects and patients with bipolar disorder we hypothesize furthermore that it might reflect a basic regulatory mechanism between serotonin transporter function and neuronal number or size in amygdala and maybe in other regions of the human brain.
As a relatively increased volume may also account for increased activity observed in functional MRI studies, our data is in accord with prior findings of higher amygdala activity in healthy carriers of the S-allele [5, 6]. By contrast, another study using voxel-based morphometry observed a reduction of amygdala gray matter volume in healthy S-allele carriers [11]. Investigation with voxel-based morphometry (VBM) allows structural analyses free from hypotheses and user-bias. Despite these advantages, its general application in structural MRI studies might be restricted by a low sensitivity in detecting marginal abnormalities. A recent study concluded that manual tracing of the corpus callosum was more sensitive in detecting discrete structural changes than VBM [15]. Therefore, the different findings in the VBM and the present study may be caused by methodological differences.
However, an increased volume of the amygdala with a higher number of neurons or an increased neuronal size could explain the observed higher activation in these subjects.
Taken together, the present findings are consistent with the view that the 5-HTTLPR polymorphism may modulate neuronal size or number in the amygdala. It seems to be worthwhile to investigate the relationship between serotonin transporter function and amygdala function and volume in further studies. Moreover, 5-HTTLPR genotype might be used as a covariate in further structural MRI investigations.
References
Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH (2005) Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord 7:570–576
Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC (2003) MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res 37:287–295
Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC (2004) Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry 56:399–405
Hariri AR, Holmes A (2006) Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci 10:182–191
Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR (2002) Serotonin transporter genetic variation and the response of the human amygdala. Science 297:400–403
Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grüsser SM, Flor H, Schumann G, Mann K, Büchel C (2005) Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8:20–21
Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, Willis MW, Danielson A, Frye MA, Herscovitch P, Post RM (2001) Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry 49:97–109
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531
McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N (2004) Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 56:411–417
Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ, Parsey RV (2007) Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry 64:201–208
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR (2005) 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834
Phillips ML, Drevets WC, Rauch SL, Lane R (2003) Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54:515–528
Scherk H, Usher J, Leucht S (2007) Amygdala volume for bipolar affective disorder—a meta-analysis. Poster at the DGPPN-Congress 20–24.11.2007. Nervenarzt 78:343
Strakowski SM, DelBEllo MP, Adler CM (2005) The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry 10:105–116
Thomann PA, Wustenberg T, Pantel J, Essig M, Schroder J (2006) Structural changes of the corpus callosum in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord 21:215–220
Weinberger DR, McClure RK (2002) Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry 59:553–558
Wittchen HU, Zaudig M, Fydrich T (1997) Strukturiertes klinisches Interview für DSM-IV Achse I und II. Hogrefe, Göttingen
Young KA, Holcomb LA, Bonkale WL, Hicks PB, Yazdani U, German DC (2007) 5HTTLPR polymorphism and enlargement of the pulvinar: unlocking the backdoor to the limbic system. Biol Psychiatry 61:813–818
Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD (2000) fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2:237–248
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Scherk, H., Gruber, O., Menzel, P. et al. 5-HTTLPR genotype influences amygdala volume. Eur Arch Psychiatry Clin Neurosci 259, 212–217 (2009). https://doi.org/10.1007/s00406-008-0853-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-008-0853-4