Abstract
Introduction
Primary squamous cell carcinoma of the parotid gland typically presents as a palpable, often painless mass. Peripheral facial palsy as the only sign of malignant neoplasia is rare. In these cases, the diagnosis is regularly confirmed by radiological imaging followed by surgical exploration and biopsy. However, if there is no detection of malignant lesions and no evidence of a tumor, the reluctance to take a biopsy of an unremarkable nerve can lead to misdiagnoses.
Case report
A 40-year-old female patient without medical history presented to our clinic with a complete right-sided peripheral facial palsy that had slowly progressed for 2.5 years. All other otorhinolaryngological examination findings were within normal limits. Magnetic resonance imaging examination of the head and neck and 18-fluorodeoxyglucose positron emission tomography showed unremarkable results. We proceeded with surgical exploration, which revealed no evidence of a tumor and an externally completely unremarkable facial nerve. A biopsy from the main trunk area of the nerve revealed an infiltration by a squamous cell carcinoma. Total parotidectomy with resection and reconstruction of the facial nerve and neck dissection was performed. Considering the absence of a primary tumor and other tumor formations the diagnosis of a completely regressive primary squamous cell carcinoma of the parotid gland was confirmed.
Conclusion
In conclusion, in the case of slow-onset peripheral facial palsy that persists without signs of recovery, a gadolinium-enhanced MRI should be performed. If imaging is unremarkable and there is no primary tumor detection along the course of the facial nerve, a surgical exploration with biopsy of the facial nerve is necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant neoplasms of the salivary glands can be classified into a variety of subtypes. Primary squamous cell carcinoma of the parotid gland represents a rare yet highly aggressive histological subtype that is often associated with a poor prognosis. Clinically, it typically presents as a palpable and often painless mass. Facial and ear pain, as well as peripheral facial palsy due to neuronal invasion and perineural spread of the malignant cells, usually develop in advanced stages [1].
Cases of slow-onset peripheral facial palsy as the only sign of malignant neoplasia of the parotid gland are rare, and can be mistaken for idiopathic facial palsy, such as Bell’s palsy [2]. Therefore, slow-onset peripheral facial palsy without signs of regeneration should be considered suspicious for malignancy until proven otherwise and requires further diagnostics.
Case report
We present the case of a 40-year-old female patient who visited our clinic complaining of slowly progressive paralysis of the right-sided facial muscles over the past 2.5 years, starting at the Mm. corrugator and orbicularis oculi, and progressing from the cranial to the caudal mimic muscles, finishing in complete facial palsy. The patient had no medical history and denied symptoms such as fever, night sweats or unintentional weight loss. Upon clinical examination, we observed a complete right-sided peripheral facial palsy (Fig. 1). All other otorhinolaryngological examination findings were within normal limits and blood tests showed unremarkable results.
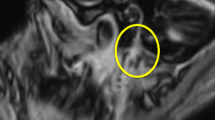
Initially, magnetic resonance imaging (MRI) examination of the head and neck soft tissues (Fig. 2), computed tomography (CT) of the petrous bone and cranial base, as well as 18-fluorodeoxyglucose positron emission tomography-computed tomography (18-FDG PET-CT) (Fig. 3), were performed. They did not detect any cause for the peripheral facial palsy. There were no observable lesions, enlarged lymph nodes or pathological 18-FDG-uptake along the course of the peripheral facial nerve. The radiological examinations were evaluated by various highly experienced radiologists in the field of head and neck imaging at our certified head and neck tumor center.
We decided to proceed with surgical exploration, which microscopically revealed no evidence of a tumor and an externally completely unremarkable facial nerve (Fig. 4). A biopsy from the main trunk area of the nerve close to its bifurcation revealed a perineural and intraneural infiltration by a p16-positive squamous cell carcinoma (Fig. 5). Following our interdisciplinary tumor board decision, a total parotidectomy with resection and reconstruction of the facial nerve using the diversification technique (auricularis magnus nerve interposition for the cranial facial nerve branches and hypoglossus-facial nerve jump anastomosis for the caudal facial nerve branches) and level II-III neck dissection were performed. After removal of the tumor-infiltrated main trunk, frozen sections were performed on the remaining nerve ends to ensure an R0 resection. Additionally, a platinum eyelid weight was implanted into the upper eyelid. The pathological examination revealed a moderately differentiated squamous cell carcinoma within the main trunk of the facial nerve without definite remnants of a primary tumor in the fully processed salivary gland (pT4a, pN0 (0/30), L0, V0, Pn1). Considering the absence of a primary tumor and other tumor formations in the otorhinolaryngological area, skin or other organs, the diagnosis of a completely regressive primary squamous cell carcinoma of the parotid gland was confirmed. Although adjuvant radiotherapy is usually performed in the case of malignant salivary gland tumor with perineural invasion, our interdisciplinary tumor board recommended a close follow-up care as an individual therapy plan due to the complete regression of the primary tumor and the sufficiently large safety margins. MRI of the soft tissues of the head and neck (after 3 months) and 18-FDG PET-CT (after 6 months) revealed no evidence of a primary tumor, locoregional lymph node involvement, distant metastases, or tumor recurrence. Whole-body CT (after 17 months) revealed no tumor regrowth or metastasis. Due to the immediate facial nerve reconstruction, the facial mimic recovered satisfactorily within 24 months, with complete eyelid closure and good facial tonus (Fig. 1).
Discussion
In the case of slow-onset, progressive peripheral facial palsy, a gadolinium-enhanced MRI should be performed to exclude a malignant neoplasm. MRI is the method of choice especially for the evaluation of tumors of the stylomastoid foramen and the retromandibular part of the parotid gland. MRI enables the detection of even small lesions in the parotid gland as well as along the entire course of the facial nerve due to its high soft tissue resolution. However, magnetic resonance imaging has shown limitations in the evaluation of perineural tumor spread causing facial palsy [3]. Therefore, a high-quality MRI should always be performed at a center with extensive experience in parotid gland pathologies and must always be interpreted in conjunction with the clinical presentation of facial palsy. In addition, 18-FDG PET-CT is known as a highly sensitive functional imaging method for the detection of malignant diseases. In some cases, however, there is no detection of malignant lesions even after extensive morphological and functional imaging. To our knowledge, the case shown here is the first case of intraneural squamous cell carcinoma of the facial nerve that showed unremarkable findings on 18-FDG PET-CT. Boahene et al. reported 15 patients with unilateral peripheral facial palsy due to occult malignancy, who showed unremarkable clinical and radiological findings (MRI and CT). A diagnosis was confirmed in 13 of these patients through exploration and biopsy of the facial nerve, with two patients requiring a second biopsy to establish malignancy [4].
Furthermore, as in the case presented here, carcinoma could not be ruled out with certainty even if surgical exploration revealed a parotid gland without any signs of a tumor and an externally unremarkable facial nerve. Eggerstedt et al. reported a case of slow-onset peripheral facial palsy due to intraneural squamous cell carcinoma, which was confirmed only by final histology [5]. Additionally, it is possible for certain areas of the nerve to be skipped during the spread of carcinoma. Therefore, obtaining a sufficiently large biopsy and performing a repeated biopsy are recommended, if a malignancy is highly suspected [2].
Conclusion
In summary, in the case of slow-onset peripheral facial palsy that persists without signs of recovery, a gadolinium-enhanced MRI should be performed. If imaging is unremarkable and there is no primary tumor detection along the course of the facial nerve, a surgical exploration with biopsy of the facial nerve is necessary. The case presented demonstrates that even with an externally unremarkable nerve, normal MRI and 18-FDG-PET-CT findings, peri- and intraneural tumor spread is possible. Resection of the parotid gland and the facial nerve is necessary when a biopsy proves malignant growth. Immediate facial nerve reconstruction allows favorable functional and esthetic recovery of the face.
References
Xiao M, Liu J, You Y, Yang X, Wang Y (2021) Primary squamous cell carcinoma of the parotid gland: clinicopathological characteristics, treatment, and prognosis. Int J Oral Maxillofac Surg 50(2):151–157. https://doi.org/10.1016/j.ijom.2020.06.010
Taga A, Di Lella F, Corcione L, Pavesi G (2017) Isolated facial palsy from perineural spread of cutaneous squamous cell carcinoma. Muscle Nerve 56(5):E40–E41. https://doi.org/10.1002/mus.25727
Jungehuelsing M, Sittel C, Fischbach R, Wagner M, Stennert E (2000) Limitations of magnetic resonance imaging in the evaluation of perineural tumor spread causing facial nerve paralysis. Arch Otolaryngol Head Neck Surg 126(4):506–510. https://doi.org/10.1001/archotol.126.4.506
Boahene DO, Olsen KD, Driscoll C, Lewis JE, McDonald TJ (2004) Facial nerve paralysis secondary to occult malignant neoplasms. Otolaryngol Head Neck Surg 130(4):459–465. https://doi.org/10.1016/j.otohns.2003.12.013
Eggerstedt M, Kuhar HN, Revenaugh PC, Ghai R, Mark Wiet R (2019) Slowly progressive facial paralysis: intraneural squamous cell carcinoma of unknown primary. Am J Otolaryngol 40(1):129–131. https://doi.org/10.1016/j.amjoto.2018.10.004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Authors have no conflict of interest to declare. No funding was received for conducting this study. All co-authors have seen and agree with the content of manuscript and there are no financial or non-financial interests that are directly or indirectly related to the work submitted for publication to report. We certify that the submission is original work and is not under review at any other publication.
Consent to publish
Patients signed informed consent regarding publishing their data and photographs.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sistori, G., Götting, M., Radke, C. et al. Biopsy of the facial nerve in slow-onset facial palsy. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08826-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08826-3