Abstract
Purpose
The intraoperative detection of cerebrospinal fluid (CSF) leaks during endoscopic skull base surgery is critical to ensure watertight sealed defects. Intrathecal fluorescein (ITF) is a valuable adjunct to intraoperative investigation. Hence, our aim is to summarize the evidence of the efficacy of ITF as an accurate diagnostic modality and reconstruction guide for non-congenital skull base defects.
Methods
Using the Cochrane Central, MEDLINE, and Embase databases, we identified studies involving the use of ITF in non-congenital CSF leaks which were published until November 2023. The STATA 18 software was used for meta-analysis.
Results
Fourteen studies met the inclusion criteria, in which seven studies were included in the meta-analysis. ITF was used in 1898 (90.3%) of patients, with a detection rate of 88.1%. The overall detection rate of non-congenital CSF leaks among ITF concentrations of 5% and 10% had a statistically significant pooled effect size of 2.6 (95% CI = 2.25, 2.95), while when comparing the ITF to other alternative radiological tests, it was not statistically significant with a mean difference of 0.88 (95% CI = − 0.4, 2.16). Moreover, the pooled prevalence was statistically significant in regards of the complications associated with ITF with an effect size of 0.6 (95% CI = 0.39, 0.82), indicating that 60% of patients who underwent ITF would experience at least one of the measured complications.
Conclusion
ITF is considered as an efficient tool in localizing skull base defects. However, there was no significant results when comparing the ITF to other alternative radiological tests. Accordingly, if the ITF intervention is indicated, patients should be carefully selected based on their clinical need.
Similar content being viewed by others
Introduction
Various methods have been implemented to improve the detection of cerebrospinal fluid (CSF) leakage sites, including intrathecal fluorescein (ITF), first described in 1960 by Kirchner and Proud [1]. Although ITF is not commonly used, it can help identify, localize, and repair CSF skull base defects [2,3,4]. The presence of green/yellow fluid delineates the CSF leak location 30 min after fluorescein administration. The sensitivity of ITF ranges from 46 to 100%, and the surgical success rate can be as high as 96% [5,6,7,8,9,10,11,12,13,14,15]. This wide range is attributed to various factors, including the fluorescein dose and concentration. For example, at a dose of 10 mg, the sensitivity of ITF is 73.8% [16], while at a dose of 25 mg, the sensitivity and specificity can reach 92.9% and 100%, respectively [17]. Despite being a desirable option when determining the extent of reconstruction, there is a lack of comprehensive systematic reviews investigating the efficacy and safety of non-FDA-approved ITF in detecting CSF leaks in non-congenital defects. Therefore, this systematic review aimed to search the literature for evidence of the importance of ITF as an accurate diagnostic modality and reconstruction guide for non-congenital skull base defects. Furthermore, we aimed to analyze the factors contributing to ITF-related complications and provide evidence-based guidelines on safe dosages and techniques.
Methods
Literature search
This systematic review followed the Cochrane review methods and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PROSPERO CRD42021273630) [18]. A literature search of the Cochrane Central, Medline, and Embase databases was conducted systematically on February 2021 and updated on November 2023, to identify articles published from 1960 onward with the following search strategy: (“CSF” [All Fields]) OR (“Cerebrospinal fluid” [All Fields]) OR (“Cerebrospinal fluid” [MeSH Terms]) AND (“Fluorescein”[All Fields]) OR (“Fluorescein” [MeSH Terms]) AND (“Intrathecal” [All Fields]) OR (“injections, spinal” [MeSH Terms]). The reference lists of the included articles were manually appraised to identify additional publications not found in the primary literature search.
Study selection
This systematic review included English and non-English studies assessing the safety and efficacy of ITF in CSF leak localization and as an intraoperative guide for skull base reconstruction. Studies met the inclusion criteria if they included skull base defects of traumatic or spontaneous etiologies repaired using endoscopic procedures. However, studies comprising congenital malformations or those who did not use ITF in diagnosing CSF leaks in any patient in their sample or those who used Extracranial approach were excluded. Nonhuman studies, review articles, narrative reviews, meta-analyses, systematic reviews, reports involving fewer than five cases, animal studies, cadaver studies, and editorials were also excluded. To meet the scope of this systematic review, three independent authors (D.R., H.J., and A.G.) screened the studies in three steps before final inclusion. When disagreements occurred, the three authors discussed the article until a consensus was reached or the decision was made based on the majority.
Data extraction
A single investigator retrieved data from a standardized database for each full-text article. General information included the first author’s name, publication year, country, study design, population size, median age, sex, and CSF leak etiology. Characteristic analyses of ITF included the preprocedural setting, fluorescein dosage and technique of administration, use of lumbar drains (LD), and detection rate. The surgical approach for reconstruction, follow-up period, ITF-related complications and CSF leak recurrence, were documented and linked to adverse outcomes. Furthermore, investigations used as an adjunct to ITF and their diagnostic rates were documented.
Quality of the studies
The quality of the included studies was assessed according to the Enhancing the Quality and Transparency of Health Research (EQUATOR) reporting statement of observational studies (STROBE) with a maximum score of 22.
Statistical analysis
In the tables, patient demographics, ITF characteristics, and outcome statistics were described using standard descriptive statistics. All fluorescein-related outcomes were reported as frequencies and percentages. The median age of the study population was reported, and the range was considered whenever the median was not mentioned. Additionally, meta-analysis was done using STATA 18 software. The models were done using random effect models to estimate the pooled log odds ratio and results were presented in forest plot. In addition, heterogeneity was tested using Cochran’s Q test and Higgins’s I2. Publication bias was investigated using funnel plot.
Results
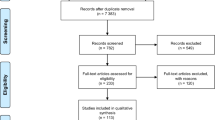
The search identified 362 unique articles. Eighty-nine studies were selected for full-text review after screening the titles and abstracts for eligibility based on the exclusion criteria. Fourteen original studies (2101 patients) were finally included after reviewing the full text; the remaining studies were excluded for reasons stated in the PRISMA diagram (shown in Fig. 1).
Study and population characteristics
The studies were either retrospective or prospective observations published between 1960 and 2023 in the United States [3, 17, 19, 20], Brazil [6, 21, 22], Germany [23, 24], India [25, 26], Italy [27], Iran [28], and Pakistan [29]. The patients were aged 1–86 years; however, the patients’ ages were unspecified in four studies. Nine studies focused primarily on ITF use [3, 6, 17, 19, 20, 22, 24, 28, 29], whereas the rest reported their surgical practice in CSF rhinorrhea management [21, 23, 25,26,27]. Of the 2101 individuals, 1898 (90.3%) had ITF as part of their management strategy for CSF leak repair. The CSF leak etiology was purely iatrogenic in Jakimovski et al. due to endoscopic pituitary surgery [19], idiopathic in the Englhard et al. cohort [23], due to skull base lesions in the Raza et al. cohort [17], and mixed traumatic and non-traumatic in the 11 remaining studies. Only six studies reported the efficacy of other diagnostic modalities, such as endoscopic inspection, beta 2-transferrin and beta trace protein, and radiological imaging [3, 21,22,23, 25, 27]. The characteristics of each study are presented in Table 1.
ITF preparation and diagnostic efficacy
Table 2 details ITF preparations and their association with positive outcomes. In most studies, ITF was injected intraoperatively after induction of anesthesia and before endoscopic exposure. Guimarães et al. and Bhalodiya et al. reported that lumbar puncture (LP) was performed before anesthesia, except in children and anxious elderly patients [6, 25]. In two studies involving 622 participants, patients were premedicated with 10 mg of intravenous dexamethasone (unless diagnosed with Cushing’s disease) and 50 mg of diphenhydramine [17, 19]. Other authors administered perioperative antibiotics to 77 patients with ITF [22, 23, 25, 26]. Although ITF preparations were notably heterogeneous among the studies, 962 of 1092 (88.1%) patients were diagnosed. CSF was the sole dilution medium used in eight studies with 791 participants. Regarding the fluorescein formula, 10 mL of CSF was mixed with 0.1–0.25 mL of 10% fluorescein in 779 participants, and it helped to diagnose 692 (88.8%) [3, 17, 19, 20]. A dose of 0.05 mL/kg to a maximum of 1.0 mL of 5% fluorescein helped diagnose 51 of 56 subjects (91.1%) [21, 23, 27, 28]. Mixing 0.1 mL of 10% fluorescein had a detection rate of up to 66.3%, compared to the 100% noted with 0.2–0.7 mL of 5% fluorescein [3, 20, 25, 26]. A German study reported that injecting 0.5–1.0 mL of 5% sodium fluorescein via LP without mixing helped diagnose 97 of 119 operated cases (81.5%) [24]. Mahmood et al. Reported the highest fluorescein formula as they used CSF of 0.1 mL mixed with 25% fluorescein which helped in diagnosing 49 patients out of 62 patients, with a detection rate of 79% [29]. In all studies, successful reconstruction of the skull base defect was achieved in 80–100% of the cases after the first intervention and 100% after the revision surgery with persistent leaks.
Follow-up and recurrence
The follow-up period ranged from 2 weeks to 106 months. CSF leak recurrence has been discussed in 11 of 14 studies involving 894 participants [17, 20,21,22,23, 25,26,27,28,29]. Only 36 patients (4%) had evidence of recurrence and were treated with revision surgery or conservatively through LD. Radabaugh et al. reported the highest recurrence rate of CSF leakage (9.9% of patients) [20]. Table 2 summarizes the recurrence rates and follow-up periods of the included studies.
ITF-related complications
Table 3 briefly summarizes the studies that assessed perioperative ITF complications. The reported complication rates of ITF injections ranged from 0 to 20%. Overall, 45 of 1,898 patients developed complications (2.4%). Seven studies reported the presence of ITF-related complications during the follow-up period [3, 19, 20, 22, 24,25,26]. Most of these complications were transient, except for one study that included a patient with permanent diplopia and 12 patients with endocrinopathies [19]. Neurological complications (e.g., seizure, meningitis, syncopal episodes, headaches, and anosmia) were the most common perioperative complications which reported in 22 patients which accounts for 48.9% of the overall complications [3, 19, 20, 22, 24, 26]. Javadi et al. reported late complications after ITF, including meningitis, pneumocephalus, and pseudoaneurysm [28]. A total of 779 subjects were injected with 0.1–0.25 mL of 10% fluorescein mixed with 9–10 mL of CSF, which was associated with complications in 31 cases (4.0%) that were primarily related to endocrinopathies [3, 17, 19, 20]. Five percent fluorescein at 0.05 mL/kg to a maximum of 1.0 mL was used in 56 participants, with complications in four cases (7.1%), all classified as late complications [21, 23, 27, 28]. In two other studies with 41 participants, 0.5–1 mL of 5% sodium fluorescein was diluted in 10 mL of distilled water and was associated with headache in only one patient (2.4%) [6, 22]. According to Wolf et al., injecting 0.5–1.0 mL of 5% sodium fluorescein through the LP without mixing resulted in seizures in three of 119 patients (2.5%) [24].
Adjunct diagnostic modalities
ITF was not used alone and was considered an adjunct to localize leaks as the authors performed diagnostic imaging on all participants. However, only seven studies reported the diagnostic rates of radiological tests (Table 4). The collective diagnostic rate of the 237 patients with alternative investigations was 75.5%, compared to 84.1% for ITF cases.
Assessment of bias
The quality assessment of each study is presented in Supplement 1. The mean score of the included studies using the STROBE statement was 15.9. The maximum score obtained was 21 of 22. Almost all studies properly elaborated on the scientific background, study design and settings, and descriptive outcome data. All studies provided a cautious overall interpretation of the results, considering the objectives and results of similar studies. The weakest aspect of the studies was that the findings were reported as descriptive statistics. Accordingly, confounder-adjusted estimates and their precision were not reported except in three studies [19,20,21]. Only three studies clearly stated the potential sources of bias [3, 17, 19].
Meta-analysis results
Seven studies have been involved in the meta-analysis out of the fourteen studies. The pooled effect size of the odds ratio for identifying non-congenital CSF leak when comparing the ITF to other alternative radiological tests (such as CT/MRI cisternogram, High-resolution Computed Tomography, and CT scan) was not statistically significant, showing a mean difference of 0.88 (95% CI = − 0.4, 2.16) with a heterogenicity of I2 = 61.17%, as seen in Fig. 2. The detection rate of non-congenital CSF leaks among ITF concentrations of 5% and 10% had a statistically significant pooled effect size of 2.6 (95% CI = 2.25, 2.95) with a heterogenicity reaching I2 = 74.98%, as seen in Fig. 3. The funnel plot in Fig. 4 illustrates the spread of study estimates in relation to the precision; however, due to the limited number of involved studies, the effectiveness of the funnel plot might be impacted. The skewed distribution of studies within the 95% CI indicates the potential for publication biases favoring the reporting of the higher ITF detection rate. Moreover, the pooled prevalence of the complications among the ITF patients was 0.6 (95% CI = 0.39, 0.82) with a heterogeneity reaching I2 = 34.24%, indicating that 60% of patients who underwent ITF would experience at least one of the measured complications, as seen in Fig. 5.
Discussion
ITF vs. radiological investigations: is ITF worth the risk?
Based on aggregated data from the studies included in this systematic review, all patients underwent radiological evaluations to diagnose CSF leakage, regardless of ITF use. High-resolution Computed Tomography was performed on all patients and was helpful in most cases, whereas some studies utilized magnetic resonance imaging (MRI) and MR/CT cisternography [30,31,32,33,34,35,36,37,38]. However, many skull base surgeons are undecided regarding whether ITF is worth the risk, considering its complications, compared to other diagnostic imaging modalities.
In a retrospective study by Flynn et al., 102 patients underwent CSF leak repair, and successful CSF leak identification was similar between preoperative CT and ITF injection when a single leak was present [3]. However, ITF had superior diagnostic ability than CT (100% vs. 81%) for multiple CSF leak sites [3]. Woodworth et al. found ITF useful in spontaneous CSF leaks, as it was superior for detecting multiple skull base defects in patients with benign intracranial hypertension [39]. This suggests the diagnostic value of ITF, irrespective of CT findings, as the presence of multiple leak sites is not always known preoperatively.
Factors affecting the efficacy of ITF
The etiology of CSF leaks can affect ITF accuracy during localization, which may be due to intrinsic differences in the patho-etiological mechanisms of the leak. For example, in idiopathic intracranial hypertension, there is a dysfunction in the CSF absorptive mechanism of the arachnoid granulations or extracranial lymphatics [40]. In patients with trauma, CSF depletion and subsequent intracranial hypotension can limit the complete dissemination and visualization of fluorescein at the skull base. Another potential explanation for traumatic leaks is the formation of leptomeningeal fibrosis due to inflammatory reactions to blood products when there is hemorrhage in the subarachnoid space. This could restrict the flow of fluorescein-stained CSF into the defect [20, 40, 41]. The ITF process may also be affected by insufficient time from intrathecal injection to endoscopic intervention or uneven fluorescein distribution within the subarachnoid space [17].
In a series by Radabaugh et al., ITF had a false-negative rate of 33.6% and was insufficient when used alone to exclude CSF leaks [20]. Raza et al. reported a much lower false-negative rate (4.5%), but they used a higher fluorescein dosage (0.25 vs. 0.1 mL of 10% fluorescein) and focused mainly on skull base lesions as an etiology [17]. If the defect cannot be localized ITF, even after head-down positioning and positive pressure ventilation maneuvers, Englhard et al. advise terminating the procedure and running additional diagnostics (e.g., CT or MRI cisternography) to guarantee accurate localization [23].
Relationship between ITF preparations and the development of complications
ITF utilization lacks Food and Drug Administration (FDA) approval and standardization, and there is no consensus among practitioners regarding its optimal utility. This poses potential risks to patients, and surgeons may face medicolegal problems [3]. Nevertheless, ITF is a second-line diagnostic tool for localizing skull base defects in operative settings and is potentially safe when used at recommended doses [3, 27, 42, 43]. Complications associated with ITF methods or doses have been reported [24, 25, 44,45,46]. High doses (500–1500 mg) are associated with various life-threatening complications, such as seizures, spinal cord injury, and even death. Although ITF has been reported to be safe at lower doses, it should be used cautiously, as a large meta-analysis by Felisati et al. reported that transient complications could occur even with ITF doses of < 50 mg [43]. Furthermore, this systematic review highlights varying preparations for ITF administration, such as hypodense fluorescein or diluting sodium fluorescein in distilled water instead of CSF. Such preparations are simpler and more effective in locating CSF fistulas, with no significant intra- or postoperative complications [22, 26, 27].
Limitations
We acknowledge the limitations of our study. First, observational studies can have unmeasured confounders in addition to the recall bias noted in retrospective cohorts. Second, the clinical and statistical heterogeneity was substantial but expected since ITF is still off-label and there are no treatment guidelines for its preparations. However, to address heterogeneity, we carefully examined the included studies for mutual variables related to our aim and used unified metric units (whenever possible) for comparison. Third, 92 patients (four studies) did not undergo ITF, and it was challenging to distinguish whether recurrent CSF leaks occurred in this group or in patients whose leaks were initially localized by ITF. However, this issue was not encountered when other complications were examined. Fourth, we acknowledge the likelihood of publication bias, along with bias in the data acquisition and review processes. The use of predetermined, strictly defined inclusion and exclusion criteria, adoption of a data extraction strategy, and robust quality assessment conducted independently by the authors indicate that this systematic review included the highest quality of evidence in reporting the efficacy and safety of the current off-label administration of ITF in diagnosing CSF leaks. Finally, the scarcity of comparative studies between ITF and other diagnostic tests affected our ability to estimate the benefit-risk ratio. However, we acquired data on the detection rates of additional diagnostic tests whenever reported to demonstrate the value of adding ITF to the management plan.
Conclusions
The effective rate of ITF for localizing CSF Leaks, reportedly having an overall detection rate of 46–100% and a surgical success rate up to 96%. Nevertheless, the surgical complication rates were reported to range from 0 to 25%. Although the need for ITF in the era of advanced radiological techniques is debated, as in our pooled results there was no significant difference in the detection rate between ITF and alternative radiological studies. Moreover, no randomized controlled trials were identified in the literature, and the heterogeneity in findings among studies means that this conclusion is supported by limited evidence. Accordingly, if the ITF intervention is indicated, Patients should be carefully selected based on their clinical need. Future studies should investigate the use of ITF combined with other diagnostic modalities to enhance its sensitivity and specificity for detecting CSF leaks.
Data availability
Data supporting this study are included within the article and supporting materials.
References
Kirchner FR, Proud GO (1960) Method for the identification and localization of cerebrospinal fluid, rhinorrhea and otorrhea. Laryngoscope 70:921–931. https://doi.org/10.1288/00005537-196007000-00004
Placantonakis DG, Tabaee A, Anand VK et al (2007) Safety of low-dose intrathecal fluorescein in endoscopic cranial base surgery. Neurosurgery 61(3):161–165. https://doi.org/10.1227/01.neu.0000289729.20083.dc
Flynn JP, Pavelonis A, Ledbetter L et al (2020) The utility of computed tomography and intrathecal fluorescein in the management of cerebrospinal fluid leak. Am J Rhinol Allergy 34(3):342–347. https://doi.org/10.1177/1945892419896243
Liu HS, Chen YT, Wang D et al (2009) The use of topical intranasal fluorescein in endoscopic endonasal repair of cerebrospinal fluid rhinorrhea. Surg Neurol 72(4):341–345. https://doi.org/10.1016/j.surneu.2009.03.034
Lund VJ, Savy L, Lloyd G, Howard D (2000) Optimum imaging and diagnosis of cerebrospinal fluid rhinorrhoea. J Laryngol Otol 114(12):988–992. https://doi.org/10.1258/0022215001904572
Guimarães RES, Becker H, Becker CG et al (2002) Localization of skull base cerebrospinal fluid leaks by using intrathecal fluorescein in a hypodense solution during surgery. Rev Bras Otorrinolaringol 68:788–791
Jones ME, Reino T, Gnoy A et al (2000) Identification of intranasal cerebrospinal fluid leaks by topical application with fluorescein dye. Am J Rhinol 14(2):93–96. https://doi.org/10.2500/105065800781692859
Virk JS, Elmiyeh B, Saleh HA (2013) Endoscopic management of cerebrospinal fluid rhinorrhea: the Charing Cross experience. J Neurol Surg B Skull Base 74(2):61–67. https://doi.org/10.1055/s-0033-1333620
Lindstrom DR, Toohill RJ, Loehrl TA et al (2004) Management of cerebrospinal fluid rhinorrhea: the Medical College of Wisconsin experience. Laryngoscope 114(6):969–974. https://doi.org/10.1097/00005537-200406000-00003
Banu MA, Kim JH, Shin BJ et al (2014) Low-dose intrathecal fluorescein and etiology-based graft choice in endoscopic endonasal closure of CSF leaks. Clin Neurol Neurosurg 116:28–34. https://doi.org/10.1016/j.clineuro.2013.11.006
Tabaee A, Placantonakis DG, Schwartz TH et al (2007) Intrathecal fluorescein in endoscopic skull base surgery. Otolaryngol Head Neck Surg 137(2):316–320. https://doi.org/10.1016/j.otohns.2006.11.012
Moseley JI, Carton CA, Stern WE (1978) Spectrum of complications in the use of intrathecal fluorescein. J Neurosurg 48(5):765–767. https://doi.org/10.3171/jns.1978.48.5.0765
Patel KS, Komotar RJ, Szentirmai O et al (2013) Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg 119(3):661–668. https://doi.org/10.3171/2013.4.JNS13124
Calcaterra TC (1985) Diagnosis and management of ethmoid cerebrospinal rhinorrhea. Otolaryngol Clin North Am 18(1):99–105
Antunes P, Perdigão M (2016) The use of intrathecal fluorescein in cerebrospinal fluid leak repair: management from an anesthesiologist’s point-of-view. Acta Anaesthesiol Scand 60(9):1323–1327. https://doi.org/10.1111/aas.12763
Seth R, Rajasekaran K, Benninger MS et al (2010) The utility of intrathecal fluorescein in cerebrospinal fluid leak repair. Otolaryngol Head Neck Surg 143(5):626–632. https://doi.org/10.1016/j.otohns.2010.07.011
Raza SM, Banu MA, Donaldson A et al (2016) Sensitivity and specificity of intrathecal fluorescein and white light excitation for detecting intraoperative cerebrospinal fluid leak in endoscopic skull base surgery: a prospective study. J Neurosurg 124(3):621–626. https://doi.org/10.3171/2014.12.JNS14995
AlEnazi A, Albar M, Alsharhan S et al (2023) The role of intrathecal fluorescein as a diagnostic modality for CSF leak localization and intra-operative guide in reconstruction. PROSPERO CRD42021273630, https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=273630 (accessed 2 January 2023)
Jakimovski D, Bonci G, Attia M et al (2014) Incidence and significance of intraoperative cerebrospinal fluid leak in endoscopic pituitary surgery using intrathecal fluorescein. World Neurosurg 82(3–4):513–523. https://doi.org/10.1016/j.wneu.2013.06.005
Radabaugh JP, Asi K, Jiang ZY et al (2022) Assessing the utility of intrathecal fluorescein in endoscopic repair of anterior skull base cerebrospinal fluid leaks. Int Forum Allergy Rhinol 12(7):967–970. https://doi.org/10.1002/alr.22947
Landeiro JA, Flores MS, Lázaro BC et al (2004) Surgical management of cerebrospinal fluid rhinorrhea under endoscopic control. Arq Neuropsiquiatr 62(3):827–831. https://doi.org/10.1590/S0004-282X2004000500016
Demarco RC, Tamashiro E, Valera FC et al (2007) Use of a hypodense sodium fluorescein solution for the endoscopic repair of rhinogenic cerebrospinal fluid fistulae. Am J Rhinol 21(2):184–186. https://doi.org/10.2500/ajr.2007.21.2972
Englhard AS, Volgger V, Leunig A et al (2018) Spontaneous nasal cerebrospinal fluid leaks: management of 24 patients over 11 years. Eur Arch Otorhinolaryngol 275(10):2487–2494. https://doi.org/10.1007/s00405-018-5089-y
Wolf G, Greistorfer K, Stammberger H (1997) Endoscopic detection of cerebrospinal fluid fistulas with a fluorescence technique. Report of experiences with over 925 cases. Laryngorhinootologie 76(10):588–594. https://doi.org/10.1055/s-2007-997486
Bhalodiya NH, Joseph ST (2009) Cerebrospinal fluid rhinorrhea: endoscopic repair based on a combined diagnostic approach. Indian J Otolaryngol Head Neck Surg 61(2):120–126. https://doi.org/10.1007/s12070-009-0049-x
Keshri A, Jain R, Manogaran RS et al (2019) Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base 80(5):493–499. https://doi.org/10.1055/s-0038-1676334
Emanuelli E, Milanese L, Rossetto M et al (2015) The endoscopic endonasal approach for cerebrospinal fluid leak repair in the elderly. Clin Neurol Neurosurg 132:21–25. https://doi.org/10.1016/j.clineuro.2015.02.013
Javadi SAH, Samimi H, Naderi F et al (2013) The use of low- dose intrathecal fluorescein in endoscopic repair of cerebrospinal fluid rhinorrhea. Arch Iran Med 16(5):264–266
Mahmood K, Shabbir Z, Ishfaq M et al (2021) Efficacy and role of intrathecal fluorescein for detection of defect site for treatment of cerebrospinal fluid rhinorrhea. Pak J Neurol Sur 25(4):519–527. https://doi.org/10.36552/pjns.v25i4.618
Farb RI, Nicholson PJ, Peng PW et al (2019) Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol 40(4):745–753. https://doi.org/10.3174/ajnr.A6016
Rajeswaran R, Chandrasekhran A, Mohanty S et al (2006) Role of MR cisternography in the diagnosis of cerebrospinal fluid rhinorrhoea with diagnostic nasal endoscopy and surgical correlation. Indian J Radiol Imaging 16:315–320
Mostafa BE, Khafagi A, Morcos JJ (2004) Combined HRCT and MRI in the detection of CSF rhinorrhea. Skull Base 14:157–162. https://doi.org/10.1055/s-2004-832259
Goodier M, Lubbe D, Andronikou S et al (2012) Spontaneous lateral sphenoid cerebrospinal fluid fistula: MRI diagnosis. SA J Radiol 16:8–10
Shetty PG, Shroff MM, Sahani DV et al (1998) Evaluation of high resolution CT and MR cisternography in the diagnosis of cerebrospinal fluid fistula. AJNR Am J Neuroradiol 19:633–639
Payne-Richard J, Frenkiel-Saul (2003) Role of computed tomographic cis- ternography in the management of cerebrospinal fluid rhinorrhoea. J Otolaryngol 32(2):93–100
Jayakumar PN, Kovoor JM, Srinkanth SG (2001) 3D steady-state MR cisternography in CSF rhinorrhoea. Acta Radiol 42(6):582–584. https://doi.org/10.1080/028418501127347412
Drayer BP, Wilkins RH, Boehnke M et al (1977) Cerebrospinal fluid rhinorrhoea demonstrated by metrizamide CT cisternography. Am J Roentgenol 129:149–151. https://doi.org/10.2214/ajr.129.1.149
Coloqhoun IR (1993) CT cisternography in the investigation of cerebrospinal fluid rhinorrhoea. Clin Radiol 47:403–408. https://doi.org/10.1016/S0009-9260(05)81061-X
Woodworth BA, Prince A, Chiu AG et al (2008) Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial hypertension. Otolaryngol Head Neck Surg 138(6):715–720. https://doi.org/10.1016/j.otohns.2008.02.010
Wall M (2010) Idiopathic intracranial hypertension. Neurol Clin 28(3):593–617. https://doi.org/10.1016/j.ncl.2010.03.003
Rhoton AL (2000) The posterior fossa cisterns. Neurosurgery 47(3):287–297
Felisati G, Bianchi A, Lozza P et al (2008) Italian multicentre study on intrathecal fluorescein for craniosinusal fistulae. Acta Otorhinolaryngol Ital 28(4):159–163
Castelnuovo P, Mauri S, Locatelli D et al (2001) Endoscopic repair of cerebrospinal fluid rhinorrhea: learning from our failures. Am J Rhinol 15(5):333–342. https://doi.org/10.1177/194589240101500509
Jolly K, Gupta KK, Banota A et al (2021) The effectiveness and safety of intrathecal fluorescein in the management of cerebrospinal fluid leaks. Am J Rhinol Allergy 35(6):879–884. https://doi.org/10.1177/19458924211020564
Keerl R, Weber RK, Draf W et al (2004) Use of sodium fluorescein solution for detection of cerebrospinal fluid fistulas: an analysis of 420 administrations and reported complications in Europe and the United States. Laryngoscope 114(2):266–272. https://doi.org/10.1097/00005537-200402000-00016
Mahaley MS, Odom GL (1968) Complication following intrathecal injection of fluorescein: case report. J Neurosurg 25:298–299. https://doi.org/10.3171/jns.1966.25.3.0298
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design: SSS, WFM, MHB, ASS, MMA, and ASE. Acquisition of data: HJJ, DFR, and AAG. Drafting the article: HJJ, DFR, and AAG. Revising the article for important intellectual content: SSS, WFM, MHB, ASS, MMA, and ASE. Approval of article and agreement for submission: SSS, WFM, MHB, ASS, MMA, ASE, HJJ, DFR, and AAG. All the co-authors listed above gave their final approval of this manuscript version.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts of interest regarding this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AlSharhan, S.S., Aljubran, H.J., Alrusayyis, D.F. et al. Diagnostic accuracy of intrathecal fluorescein versus other radiological modalities in evaluating non-congenital skull base defects: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08603-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08603-2