Abstract
Purpose
Computed tomography (CT)-defined sarcopenia, as a measurement of low skeletal muscle (SM), is a poor prognostic indicator in patients with head and neck cancer (HNC), independent of weight or nutritional status. We used SM measures at the second thoracic vertebra (T2) to determine T2-SM index (SMI) thresholds for sarcopenia, and investigate the impact of low T2-SMI on overall survival (OS), and weight loss during radiotherapy (RT).
Methods
Adult patients with newly diagnosed HNC with a diagnostic PET–CT or RT planning CT scan were included. SM was analysed at T2 and a model applied to predict SM at L3. T2-SMI thresholds for sarcopenia were established with predicted measures, stratified by BMI and sex. Impact of sarcopenia and low T2-SMI on OS and weight loss during RT was investigated.
Results
A total of 361 scans were analysed (84% males, 54% oropharynx tumours). Sarcopenia was found in 49%, demonstrating worse OS (p = 0.037). T2-SMI cutoff values were: females—74 cm2/m2 [area under the curve (AUC): 0.89 (95%CI 0.80–0.98)], males (BMI < 25)—63 cm2/m2 [AUC 0.93 (95%CI 0.89–0.96)], males (BMI ≥ 25)—88cm2/m2 [AUC 0.86 (95%CI 0.78–0.93)]. No difference in OS with T2-SMI categories. Lowest T2-SMI quartile of < 63 cm2/m2 demonstrated worse OS (p = 0.017). Weight loss during RT was higher in patients; who were not sarcopenic (6.2% vs 4.9%, p = 0.023); with higher T2-SMI (6.3% vs 4.9%, p = 0.014) and; in the highest quartiles (3.6% vs 5.7% vs 7.2%, p < 0.001).
Conclusions
These T2-SMI thresholds are effective in assessing CT-defined sarcopenia in HNC. Further assessment of clinical application is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of head and neck cancer (HNC) worldwide is increasing, with more than 650,000 cases annually and 380,000 deaths [1]. In Australia, in 2019, there were over 5000 reported cases [2]. Malnutrition has been reported in up to 70% of patients with HNC [3,4,5,6], manifesting as a multifactorial syndrome due to a combination of inadequate oral intake and metabolic derangements, caused by the tumour itself, and/or the impact of treatment-related toxicities [7, 8]. One of the phenotypic diagnostic criteria for malnutrition, as recommended by the Global Leadership Initiative on Malnutrition (GLIM) is reduced skeletal muscle mass [9], with methods of evaluation making opportunistic use of diagnostic computed tomography (CT) images in patients with cancer. Low muscle mass, quantified with CT images as skeletal muscle index (SMI), is known as CT-defined sarcopenia, the prevalence of which has been demonstrated to be a poor prognostic indicator, independent of weight or nutritional status [10,11,12], especially in patients with HNC [13, 14]. The incorporation of body composition measures, especially skeletal muscle mass assessment, in patients with HNC allows for more robust nutritional screening at the time of diagnosis.
The most widely used method for CT skeletal muscle analysis is assessment of the cross-sectional area (CSA) of an axial slice at the level of the third lumbar vertebra (L3), as muscle at this level has been shown to best represent whole body measures [15, 16]. However, abdominal scans with the L3 level visible may not be readily available in patients with HNC. Alternative anatomical landmarks have been used in many recent studies, some yet to be validated, and with varying results. The most commonly used alternative in HNC is the third cervical vertebra (C3), with some researchers also choosing thoracic options [17]. Many of these studies have formulated prediction models to estimate L3-CSA using measures at other vertebral levels. Our group has discovered recently that the most commonly used prediction model applying CSA measures at C3 demonstrated weak agreement with actual measures in our Australian cohort of majority overweight or obese patients with HNC [18]. Positioning of the neck, the presence of tumour, and concern surrounding the appropriateness of muscles in the neck to predict musculature in the abdomen, or whether muscle wasting occurs in similar proportions, are all issues that caution the use of muscle measures at this level [19]. Assessment of muscle at the thoracic level may be more feasible due to the larger muscle groups present that may be more susceptible to muscle wasting, and we have previously shown that measures taken at the second thoracic level (T2) correlate well to measures at L3 [20]. Pai et al. measured adipose tissue and skeletal muscle at T2 and suggested cutoff values based on sex-specific median values in a Taiwanese population, finding that skeletal muscle measures have no significant impact on overall survival (OS) [21]. However, theses ranges are very population-specific and not translatable to our Australian cohort. At present, there are no HNC-specific cutoff values for sarcopenia that are applicable across populations.
The aim of this study was to determine SMI cutoff values for sarcopenia using T2-CSA measures in our cohort of patients with HNC, and to determine the association between T2-measured low SMI and OS, and weight loss during treatment.
Materials and methods
This is an Ethics approved (2019/ETH13149), single-institution, retrospective cohort study investigating CT-defined sarcopenia in all newly diagnosed adult patients (≥ 18 years) with HNC who presented to our facility between January 2005 and February 2022. Inclusion criteria were patients with; pathology-confirmed squamous cell carcinoma of the larynx, hypopharynx, oropharynx, nasopharynx or oral cavity and a diagnostic PET–CT scan or radiotherapy (RT) planning CT scan. Patients were excluded if they had history of a previous cancer diagnosis, or had an unusable CT scan. “Unusable” CT scans were classified as those images with low resolution, overt skewing of the humeral heads at the level of T2, or if any musculature (such as the deltoids) were outside the frame of the scan. Patient baseline and treatment characteristics were retrieved from electronic and written patient records. Weights and heights were recorded at the time of PET–CT scan or at the time of attendance to the head and neck clinic. In patients who completed treatment, weights were also recorded in the final week of radiotherapy and percentage weight loss calculated.
Skeletal muscle analysis
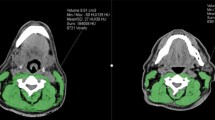
All suitable PET–CT scans and RT planning CT scans were anonymised and assessed for skeletal muscle CSA at the level of T2 via manual delineation by a single observer (trained in the Alberta Protocol with a 1.1% interrater variation achieved) (BV) with landmarking accuracy supervised by a Senior Radiologist (DM). Slice-O-Matic Version 5.0 (Tomovision, Montreal, Canada) was used for muscle delineation, with skeletal muscle identified with standard Hounsfield Units (HU) of − 29 to + 150 HU [22, 23]. Landmarking of the axial slice to be analysed at T2 utilised previously described methodology [20], where the vertebral level was selected by scrolling from a cephalad to caudal direction from the top of the second thoracic vertebra, four slices from the top, in scan slices of 3 mm thickness. All large muscle groups visible in the scan were tagged and included: the sternocleidomastoid, pectoralis major and minor, deltoid, trapezius, subscapularis, rhomboid major, infraspinatus, splenius cervicis, and multifidus muscles, with smaller, less clearly defined muscles not analysed.
Skeletal muscle at L3 was also analysed in patients who had a PET–CT to test T2-SMI values against actual L3-SMI measures and compare to cutoff values determined with predicted L3-SMIs. CSA at L3 was measured using previously defined methodology [22, 23].
Sacropenia assessment
Skeletal muscle T2-CSA was recorded and the previously described prediction model[20] was applied to convert the values into predicted values at L3;
(For Sex use: 1 for male, 2 for female)
These predicted L3-CSA measures were normalised for stature and converted into SMI for each patient (cm2/height2). Patients were categorised by body mass index (BMI, kg/m2) as: underweight (BMI < 20.0), healthy weight (BMI 20.0–25.0), overweight (BMI 25.0–29.9) or obese (BMI ≥ 30.0). BMI measures were used to categorically classify patients as being sarcopenic as per previously defined BMI and sex-specific thresholds for females of SMI < 41 cm2/m2, and in males < 43 cm2/m2 (if underweight or healthy weight) and < 53 cm2/m2 (if overweight or obese) [24].
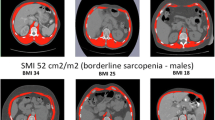
T2-CSA was also normalised for stature and converted to a measure of T2-SMI. The T2-SMI measures were used to determine corresponding cutoff values for sarcopenia classification. The whole cohort was used for T2-SMI cutoff analysis.
Weight loss during radiotherapy
A sub-set of the cohort had completed radiotherapy (± other modalities) with curative intent and were included for weight loss analysis. Patients who did not complete prescribed treatment modality were excluded. The outcome measure was percentage weight loss during treatment. Total percentage weight loss was determined by subtracting the patients’ weight in the final week of radiotherapy from their initial weight, and converting to a percentage value. Percentage weight loss was compared amongst those with sarcopenia and those with low T2-SMI. Critical weight loss (CWL) was defined as a loss of ≥ 5%. The insertion of feeding tubes was noted and investigated in terms of T2-SMI category and CWL. Prophylactic tubes were defined as those inserted prior to treatment and reactive inserted during or post-RT completion.
Survival
OS was defined as death from any cause and was calculated from the date of diagnostic or radiotherapy planning scan until date of death. Patients were censored if they were alive at the last date of follow-up. Comparison of sarcopenia status, T2-SMI cutoff values, and T2-SMI quartiles were investigated for association with OS.
Statistical analysis
All analyses were performed using SPSS Statistics software package, Version 27.0 (IBM, Armonk, NY), with statistical significance set at p < 0.05 and all p values were two-sided. Descriptive continuous variable statistics are presented as mean ± standard deviation (SD) and normality determined using Shapiro–Wilk test. All categorical data are expressed as frequencies and percentages. Receiver Operator Characteristic (ROC) tests were performed on three groups (females, males BMI < 25 kg/m2 and males BMI ≥ 25 kg/m2), to determine sex and BMI-specific T2-SMI cutoff values based on sarcopenia status. The area under the curve (AUC), sensitivity, and specificity of the cutoff values are described, and the Youden index was used to identify the optimal T2-SMI cutoff values for each patient group. Patients were classified as having a “low” T2-SMI below the cutoff. T2-SMI quartile values were also compared to determine the cutoff point with the highest risk of sarcopenia, i.e., those with the lowest T2-SMI measures. This was also tested using the actual L3-SMI measurements in the sub-set of patients with a PET–CT scan (n = 111). Chi-squared test was applied to determine the sensitivity and specificity of the T2-SMI measures compared to sarcopenia. Clinical and disease predictors of sarcopenia were analysed using binary logistic regression, with multivariate models applying both T2-SMI and T2-SMI cutoff categories separately as potential predictors.
Weight loss percentages in patients with or without sarcopenia and those with low T2-SMI or not were compared using independent samples T tests. One-way ANOVA was used to investigate percentage weight loss differences between T2-SMI quartiles. Kaplan–Meier curves were used to visualise OS based on sarcopenia status and T2-SMI, and compared using the log-rank test. Univariate and multivariate analysis was conducted using Cox regression modelling to investigate the association of baseline characteristics on OS. The backward conditional method was applied, with a significance of p < 0.2 as criteria for variable inclusion in the multivariate model. Hazard ratios and 95% confidence intervals (CI) are provided.
Results
A total of 530 patients had a diagnostic PET–CT scan or a radiotherapy planning scan between 2005 and 2022, of which 414 had a usable T2 axial slice. Fifty-three patients were excluded due to skewed shoulders or missing heights. Therefore, a total of 361 patient scans were included in this study, with this cohort used for T2-SMI cutoff analysis. Only 335 patients had completed radiotherapy (± other modalities), and were used for outcome investigations. Patient characteristics are displayed in Table 1. The majority of patients were male (84%), the largest tumour group was oropharynx carcinoma (54%) and of those patients who completed treatment (n = 335), the majority had concurrent chemoradiotherapy (51%).
A total of 175 (49%) patients were sarcopenic using the prediction model for determining L3-CSA, and application of the previously described thresholds [24]. Sixty-five percent (n = 113) of these patients had sarcopenic obesity. Kaplan–Meier survival curves showed patients who were sarcopenic at diagnosis had worse OS (Log Rank p = 0.037) (Fig. 1), with 1-year survival rates of 81% vs 87%, and 5-year rates of 62% vs 76% when compared to those who were not sarcopenic. Median survival was 8.5 vs 9.4 years (95% CI 7.5–9.6).
The cutoff values for low T2-SMI were categorised into three sex and BMI-specific groups. The ROC curves are displayed in Fig. 2, with all AUC values > 0.85 (considered to be good, and > 0.9 excellent). The T2-SMI cutoff values were; females (regardless of BMI), − 74 cm2/m2, and males: BMI < 25 kg/m2—63 cm2/m2 and BMI ≥ 25 kg/m2—88 cm2/m2. The sensitivity and specificity values with confidence intervals (CI) are shown in Table 2. Application of these cutoff values found that 181 (50%) patients had low T2-SMI. When patients were categorised as high or low T2-SMI, the comparison to corresponding sarcopenia diagnosis demonstrated a sensitivity of 80% and specificity of 82%. Testing of cutoff values using actual measures at L3 in patients with a PET–CT determined similar values to predicted measure (supplemental material, Fig. 4). Low T2-SMI was found to be a predictor of sarcopenia on multivariate logistic regression analysis (HR 67.28, CI 27.59–164.08, p < 0.001) along with sex (female, HR 10.27, CI 3.65–28.92, p < 0.001) and increasing age (HR 1.17, CI 1.11–1.22, p < 0.001) (Table 3). In separate analysis with the continuous T2-SMI variable, a lower T2-SMI value increased the risk of being sarcopenic (HR 0.93, CI 0.91–0.95, p < 0.001).
Kaplan–Meier survival curves with T2-SMI categories did not show a significant difference in survival in those with low T2-SMI (Fig. 3). The lowest 25th percent quartile for the cohort was 63 cm2/m2, and the 75th quartile was 94cm2/m2. The OS curve comparing three quartile groups (low, moderate and high) is also shown in Fig. 3, demonstrating that the low threshold of 63 cm2/m2 in the whole cohort, regardless of sex or BMI, was associated with worse OS (Log Rank p = 0.017). This translated to a 1-year survival rate of 73% vs 84% (moderate T2-SMI quartile) vs 95% (high T2-SMI quartile), and 5-year rate of 56% vs 70% vs 81%. Median survival in the low T2-SMI quartile was 7.6 vs 9.6 vs 9.0 years (95% CI 6.17–9.1).
In the sub-set of patients who completed RT, cox regression analysis indicated that on a univariate level, several patient and disease factors were associated with OS, including sarcopenia and low quartile T2-SMI. However, on multivariate analysis, only males, increasing age, and later stage disease (T3, T4 and N3) retained significant association with OS (Table 4).
Patients who presented without sarcopenia had a significantly higher mean percentage weight loss during treatment (6.2% vs 4.9%, p = 0.023). This was also observed in those patients with high T2-SMI (6.3% vs 4.9%, p = 0.014), and in the higher T2-SMI quartile ranges (3.6% vs 5.7% vs 7.2%, p < 0.001). Sixty percent of these patients experienced CWL (n = 202), with a third of these presenting with baseline sarcopenic obesity (n = 67). A significantly higher proportion of patients with mod–high T2-SMI experienced CWL (p = 0.011). Reactive enteral feeding tubes were required in 31% of patients experiencing CWL, in a significantly higher proportion than the rest of the cohort (p < 0.001). T2-SMI and weight loss category patient characteristics are shown in Table 5. ROC curves were tested with actual L3 measures, with OS and CSS curves displayed in supplementary material (Figs. 5, 6).
Discussion
This is the first study to our knowledge to investigate the use of skeletal muscle at T2 for the development of SMI cutoff values for CT-defined sarcopenia to determine association with OS and weight loss outcomes in patients with HNC. We present sex and BMI-specific cutoff values of T2-SMI for sarcopenia risk stratification in patients with HNC that can be applied when the L3 landmark is not available.
Although the sex and BMI-specific T2-SMI cutoff values did not translate into a significant association with OS, in contrast to the predicted sarcopenia measures, survival was lower in those below the set cutoff values. This is consistent with Pai et al. who also found no relation [21]. There was an association with worse OS in those patients below the 25th percentile threshold regardless of sex or BMI. Quartile thresholds, however, should be used with caution as they may not be a clear indication of true prognostic value [25]. Prediction modelling demonstrated T2-SMI to be predictive of sarcopenia in our cohort when applying our cutoff values, suggesting they are useful in identifying patients at risk of sarcopenia, and can be applied clinically as part of overall patient nutritional assessment. Patients with a T2-SMI below 63 cm2/m2 may be at the greatest risk with the demonstrated association with OS.
Our findings on the association of sarcopenia and OS are consistent with the two recent meta-analyses in patients with HNC. Wong et al., demonstrated CT-defined sarcopenia was associated with a significantly worse OS in eight studies [14] and Findlay et al., identified that pre and post-treatment sarcopenia were both independent poor prognostic indicators in patients undergoing radiotherapy treatment in six studies [13]. Of note, however, is the heterogeneity of tumour sites used in these studies and the varying vertebral levels and sarcopenia cutoff values used.
The T2-SMI cutoff values in the present study were determined with ROC curves based on sarcopenia assessment using predicted L3-SMI, with BMI and sex stratifications. The sarcopenia cutoff values suggested by Martin et al. [24] applied for this categorisation are not based on a HNC population. As specific cutoff values for sarcopenia in HNC have yet to be established, these were deemed the most closely representative of our population and have been previously applied in an Australian cohort [26]. However, this may have an impact on our final findings. We note that those cutoff values are survival-based, and generated from a heterogeneous sample of patients with respiratory and gastrointestinal tumours, who have different survival rates, and may impact on the outcomes of our study. Results may also have been influenced by the percentage error in the prediction of values at L3. However, testing conducted on the sample cohort using actual L3 measures found similar corresponding T2-SMI cutoff values and the AUC was high in all groups indicating good discriminative power for the low T2-SMI values.
Outcome-based low skeletal muscle cutoff values for patients with HNC, e.g., risk of toxicity, dysphagia, post-operative complications, survival etc., have been suggested by several authors, mostly using the C3 vertebral landmark and presenting cutoff values in terms of predicted measures at L3. However, several limitations should be noted in these studies, namely, that; most lack sex-specific stratification [27,28,29,30,31], or have used population-specific median, mean or percentile values [21, 32,33,34], or have been extracted from populations that differ in ethnicity [21, 28, 35] (and, therefore, average body sizes/BMIs), deeming suggested cutoff values difficult to transfer across populations. Recently, Zwart et al. suggested sex-specific low C3-SMI values for risk of radiotherapy toxicities in a similar, yet smaller cohort (n = 196), applying the ROC curve method; however, all AUC values were poor at < 0.7 [36]. Similarly, Galli et al. applied ROC curves for low cervical SMM cutoff values, again with low AUCs < 0.63 [37]. The difficulty with establishing universal cutoff values in HNC is the heterogeneity of patients, with differing presentation characteristics for each specific tumour site, and studies investigating different cohorts of tumours of the head and neck (e.g., inclusion of salivary gland or nasal cavity tumours). Survival also differs, especially with the inclusion of patients with small, early stage laryngeal tumours, and oropharyngeal carcinomas, as those with human papillomavirus-positive disease, for example, demonstrate better survival rates [38]. Toxicity-related weight and muscle loss can also be tumour-site dependent as treatment varies; therefore, sarcopenia cutoff values for HNC may be more applicable if they were sex, ethnicity, and tumour site-specific.
In several studies using C3, cutoff values are provided as a predicted value at L3, via the application of the Swartz et al. [39] prediction model [27, 29, 36]. Our group has demonstrated this method lacked clinically acceptable agreement with L3 measures in our population [18], and therefore, we applied our prediction model for T2-CSA conversion in this study [20]. The ROC curves generated in the present study using our predicted measures and T2-SMI had high sensitivity and specificity levels of over 70%. This demonstrates that SMI measures derived from skeletal muscle at T2 are a feasible option for muscle mass assessment in this population.
Thoracic skeletal muscle assessment has been investigated previously in HNC. Pai et al. (n = 398) used median T2-SMI sex-specific cutoff values which were considerably lower than found in our study; females < 34.3 cm2/m2 vs < 74 cm2/m2 and males < 51.74 cm2/m2 vs < 66 cm2/m2 and < 88 cm2/m2) [21]. Matsuyama et al. suggested a prediction model using T12 in a Japanese population [40], deemed unsuitable to apply to our cohort due to the differences in ethnicity and T12 not being visible in a head and neck scan. We chose T2 as this musculature is associated with muscle wasting, and is assessed as part of the physical examination of the Subjective Global Assessment, used to diagnose malnutrition [41, 42]. This level is visible in a head and neck CT scan and a radiotherapy planning scan, increasing the accessibility for clinical application.
CWL loss during treatment has been shown to negatively impact both patient survival and the ability to tolerate treatments [3, 43, 44]. We have demonstrated that patients with higher T2-SMI experienced significantly higher percent weight loss during treatment. Our Australian cohort is typical of a western population, having the majority of patients classified as overweight or obese. Approximately two-thirds of the cohort in this study presented as overweight or obese, and 70% of these patients lost ≥ 5% of their body weight during radiotherapy (the majority having a mod–high T2-SMI). This could explain the difference in weight loss, as patients who present as overweight or obese, may not have early nutritional intervention if there are no visible signs of wasting or malnutrition, due to a misconception that they have adequate muscle stores. Reactive feeding tubes are often inserted when high percentage weight loss has occurred, and in this cohort, more patients with mod–high thoracic muscle stores required this intervention.
Prado et al. demonstrated sarcopenia in obese patients is independently associated with worse survival [45], and without body composition assessment prior to treatment commencement for HNC, high risk patients may be overlooked for early interventions. A small proportion of patients who were either overweight or obese and sarcopenic (12%), had high T2-SMI measures. This represents a cohort of patients for which the application of the T2-SMI cutoff values in this study alone may not identify all patients with sarcopenic obesity. Nutritional assessments in clinical practice should incorporate several screening measures, as one parameter alone may not be an indicator of nutritional risk.
This small, single-centre retrospective study does have some limitations. The number of patients excluded due to “unusable” scans was considerable, and may have impacted on overall findings and created a bias. Ideally, prospective scan analysis, ensuring that all muscles at T2 are visible and patient positioning is optimum, would enable more robust investigation. The T2-SMI cutoff values have been determined through the application of a prediction model to estimate L3-SMI for sarcopenia stratification, and should be validated against true measures at L3. However, at our Cancer Centre, as with many internationally, it is not standard practice for all patients with HNC to have a PET–CT scan that would have L3 visible. Consequently, this adds to a cohort number that, although substantial, may not have been adequate to demonstrate definitive outcomes with T2-SMI application. HNC patient numbers are relatively small in Australian Centres, and a multi-centre collaborative approach is required for future research and validation of T2. The heterogeneous HNC sites in this cohort should also be considered and may affect applicability across populations. Tumour-site specific SMI thresholds are likely required. This study used CT muscle measures to define sarcopenia. Measures of muscle strength and function should ideally be incorporated into future research for thorough investigation of muscle loss outcomes in HNC.
In this study, we have suggested sex and BMI-specific T2-SMI cutoffs that are associated with sarcopenia in patients with HNC, and also a low T2-SMI value from the lowest quartile of the cohort to identify those at highest risk of worse OS.
Conclusions
T2 is a clinically relevant, readily accessible vertebral level in a head and neck CT scan or radiotherapy planning scan, and provides a larger group of skeletal muscles to assess for depletion in patients with HNC. Application of the suggested cutoff values for sarcopenia detection can be used for risk screening and appropriate and timely nutritional intervention.
Data availabiltiy
No additional data are available.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Australian Institute of Health and Welfare (2019) Cancer in Australia 2019. AIHW, Canberra
Alshadwi A, Nadershah M, Carlson ER, Young LS, Burke PA, Daley BJ (2013) Nutritional considerations for head and neck cancer patients: a review of the literature. J Oral Maxillofacial Surg 71:1853–1860
Mulasi U, Vock DM, Jager-Wittenaar H, Teigen L, Kuchnia AJ, Jha G et al (2020) Nutrition status and health-related quality of life among outpatients with advanced head and neck cancer. Nutr Clin Prac 35:1129–1137
Jager-Wittenaar H, Dijkstra P, Dijkstra G, Bijzet J, Langendijk J, van der Laan B et al (2017) High prevalence of cachexia in newly diagnosed head and neck cancer patients: an exploratory study. Nutrition 35:114–118
Mulasi U, Vock DM, Kuchnia AJ, Jha G, Fujioka N, Rudrapatna V et al (2018) Malnutrition identified by the Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition Consensus Criteria and Other Bedside Tools is highly prevalent in a sample of individuals undergoing treatment for head and neck cancer. J Parenter Enteral Nutr 42:139–147
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495
Baracos V, Martin L, Korc M, Guttridge D, Fearon KCH (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4:17105
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T et al (2019) GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr 38:1–9
Martin L, Gioulbasanis I, Senesse P, Baracos V (2020) Cancer-associated malnutrition and CT-defined sarcopenia and myosteatosis are endemic in overweight and obese patients. J Parenter Enteral Nutr 44:227–238
Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado C (2016) Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc 75:199–211
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67
Findlay M, White K, Stapleton N, Bauer J (2021) Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin Nutr 40:1711–1718
Wong A, Zhu D, Kraus D, Tham T (2021) Radiologically defined sarcopenia affects survival in head and neck cancer: a meta-analysis. Laryngoscope 131:333–341
Shen W, Punyanitya M, Wang Z, Gallagher D, St.-Onge M, Albu J et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338
Prado C, Heymsfield S (2014) Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enteral Nutr 38:940–953
Vangelov B, Bauer J, Kotevski D, Smee R (2022) The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr 127:722–735
Vangelov B, Bauer J, Moses D, Smee R (2022) The effectiveness of skeletal muscle evaluation at the third cervical vertebral level for computed tomography-defined sarcopenia assessment in patients with head and neck cancer. Head Neck 44:1047–1056
Vangelov B, Bauer J, Moses D, Smee R (2022) Comparison of skeletal muscle changes at three vertebral levels following radiotherapy in patients with oropharyngeal carcinoma. Nutr Cancer 75:572–581
Vangelov B, Bauer J, Moses D, Smee R (2023) The use of the second thoracic vertebral landmark for skeletal muscle assessment and CT-defined sarcopenia evaluation in patients with head and neck cancer. Head Neck 45:1006–1016
Pai PC, Chuang CC, Chuang WC, Tsang NM, Tseng CK, Chen KH et al (2018) Pretreatment subcutaneous adipose tissue predicts the outcomes of patients with head and neck cancer receiving definitive radiation and chemoradiation in Taiwan. Cancer Med 7:1630–1641
Heymsfield S, Wang Z, Baumgartner R, Ross R (1997) Human body composition: advances in models and methods. Annu Rev Nutr 17:527–558
Mitsiopoulos N, Baumgartner R, Heymsfield S, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122
Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Altman DG, Lausen B, Sauerbrei W, Schumacher M (1994) Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. JNCI J Natl Cancer Inst 86:829–835
Findlay M, White K, Brown C, Bauer J (2021) Nutritional status and skeletal muscle status in patients with head and neck cancer: Impact on outcomes. J Cachexia Sarcopenia Muscle 12:2187–2198
Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ et al (2017) Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol 71:26–33
Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY (2019) Efficacy of head and neck computed tomography for skeletal muscle mass estimation in patients with head and neck cancer. Oral Oncol 95:95–99
Chargi N, Bril SI, Swartz JE, Wegner I, Willems SM, de Bree R (2020) Skeletal muscle mass is an imaging biomarker for decreased survival in patients with oropharyngeal squamous cell carcinoma. Oral Oncol 101:104519
Huiskamp LFJ, Chargi N, Devriese LA, de Jong PA, de Bree R (2020) The predictive and prognostic value of low skeletal muscle mass for dose-limiting toxicity and survival in head and neck cancer patients receiving concomitant cetuximab and radiotherapy. Eur Arch Otorhinolaryngol 277:2847–2858
Karsten RT, Al-Mamgani A, Bril SI, Tjon-A-Joe S, van der Molen L, de Boer JP et al (2019) Sarcopenia, a strong determinant for prolonged feeding tube dependency after chemoradiotherapy for head and neck cancer. Head Neck 41:4000–4008
Karavolia E, van Rijn-Dekker MI, Van den Bosch L, van den Hoek JGM, Oldehinkel E, Meijer TWH et al (2022) Impact of sarcopenia on acute radiation-induced toxicity in head and neck cancer patients. Radiother Oncol 170:122–128
van Rijn-Dekker MI, van den Bosch L, van den Hoek JGM, Bijl HP, van Aken ESM, van der Hoorn A et al (2020) Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother Oncol 147:103–110
Sealy MJ, Dechaphunkul T, van der Schans CP, Krijnen WP, Roodenburg JLN, Walker J et al (2020) Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin Nutr 39:501–509
Lin SC, Lin YS, Kang BH, Yin CH, Chang KP, Chi CC et al (2020) Sarcopenia results in poor survival rates in oral cavity cancer patients. Clin Otolaryngol 45:327–333
Zwart AT, Pörtzgen W, van Rijn-Dekker I, Sidorenkov GA, Dierckx RAJO, Steenbakkers RJHM et al (2022) Sex-specific cut-off values for low skeletal muscle mass to identify patients at risk for treatment-related adverse events in head and neck cancer. J Clin Med 11:4650
Galli A, Colombo M, Prizio C, Carrara G, Lira Luce F, Paesano PL et al (2022) Skeletal muscle depletion and major postoperative complications in locally-advanced head and neck cancer: a comparison between ultrasound of rectus femoris muscle and neck cross-sectional imaging. Cancers 14:347
D’Souza G, Anantharaman D, Gheit T, Abedi-Ardekani B, Beachler DC, Conway DI et al (2016) Effect of HPV on head and neck cancer patient survival, by region and tumor site: a comparison of 1362 cases across three continents. Oral Oncol 62:20–27
Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KMA, de Bree R et al (2016) Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol 62:28–33
Matsuyama R, Maeda K, Yamanaka Y, Ishida Y, Kato R, Nonogaki T et al (2021) Assessing skeletal muscle mass based on the cross-sectional area of muscles at the 12th thoracic vertebra level on computed tomography in patients with oral squamous cell carcinoma. Oral Oncol 113:105126
Ottery F (2000) Patient-generated subjective global assessment. In: McCallum P, Polisena C (eds) The clinical guide to oncology nutrition. The American Dietetic Association, Chicago, pp 11–23
Jager-Wittenaar H, Ottery F (2017) Assessing nutritional status in cancer: role of the Patient-Generated Subjective Global Assessment. Curr Opin Clin Nutr Metab Care 20:322–329
Langius J, Bakker S, Rietveld D, Kruizenga H, Langendijk J, Weijs P et al (2013) Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer 109:1093–1099
Vangelov B, Kotevski D, Smee R (2021) The impact of critical weight loss and reactive feeding tubes on cancer-specific survival in head and neck cancer. Nutr Cancer 73:262–272
Prado C, Lieffers J, McCargar L, Reiman T, Sawyer MB, Martin L (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Support was provided for this work by an Australian Government Research Training Program Scholarship as part of a PhD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vangelov, B., Smee, R., Moses, D. et al. Thoracic skeletal muscle index is effective for CT-defined sarcopenia evaluation in patients with head and neck cancer. Eur Arch Otorhinolaryngol 280, 5583–5594 (2023). https://doi.org/10.1007/s00405-023-08162-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-08162-y