Abstract
Purpose
The porcine model has been demonstrated to be cost-effective for head and neck surgery training. There is no literature describing the porcine head and neck anatomy. The purpose of this study is to provide a porcine surgical guide for training head and neck residents.
Methods
Five head and neck dissections were performed under general anesthesia on the Large White pig model in the animal facilities of the University Hospital Fundación Jiménez. Sessions were photographed, and reference anatomical measurements were taken.
Results
The sternum–chin distance (x = 15.80 cm, σ = 0.44), chin–chin distance (x = 11.10 cm, σ = 2.30), prelaryngeal musculature length (x = 10.30 cm, σ = 1.92) and supraomohyoid triangle area (x = 7.07 cm2, σ = 3.91) were among the measurements obtained. The porcine head and neck anatomy was detailed.
Conclusions
Head and neck porcine anatomy was thoroughly described, with emphasis on the similarities with human anatomy. The porcine model is capable of simulating human anatomy for surgery training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A challenging learning curve demands specialized training for procedures like tracheotomy, neck dissection and laryngectomy. Head and neck (H&N) surgery is becoming less crucial in some cases due to the introduction of radiation therapy, chemotherapy, and immunotherapy in favor of organ preservation strategies [1]. Due to the greatly reduced number of surgeries that residents in otolaryngology and head and neck surgery can attend, this has a considerable impact on their training. Residents can overcome this problem with the aid of simulation models, which are now receiving more attention from researchers across all surgical specialties, not just in otolaryngology [2,3,4,5].
Despite the fact that the porcine model has historically been used as a surgical training tool to improve skills in H&N [6], there is no established approach or implementation methodology for its use in H&N resident training, and it is still being validated.
Amidst the number of publications that discuss how well the porcine model works for various procedures we have not been able to locate a guidebook that explains swine neck anatomy specifically for use in head and neck surgery simulation. Propst et al. [7] detailed manual on handling pigs and instruction in airway surgery was released in 2010; nevertheless, laryngectomy and neck dissection were not included.
We questioned how an otolaryngology resident might start his training in H&N surgery when the majority of operations are difficult cases nowadays (radiated patients, salvage surgery, second tumors, or relapses). In an effort to find a solution, we began developing the live porcine model in 2015 [8]. However, the lack of a trustworthy anatomical guideline presented several challenges.
The goal of this paper is to provide a guide for surgical dissection in the porcine model that allows for the transfer of skills learned in the porcine model to head and neck surgery in humans, with a focus on procedures such as tracheotomy, neck dissection, and total laryngectomy.
Materials and methods
Project approval
The IIS-FJD Ethical Committee and the Animal Welfare Committee of the Fundación Jiménez Díaz University Hospital gave their approval to the conducted study. The method for handling animals was inspired by a prior study from our team [8], which discussed and provided details on the progress of the resident skills following training with a live swine model.
Animal model
Five large white pigs served as models. The animals were between three and six months old and weighed between 20 and 25 kg. A tracheal tube was utilized for ventilation after 5% isoflurane was administered to anesthetize each specimen. An overdose of sodium thiopental was administered to the animal for euthanasia at the conclusion of the surgical simulation session. Adequate measures were taken to minimize pain and discomfort in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and in accordance with local laws and regulations.
Data
Table 1 displays the anatomical measurements made while dissecting the pig model. Additionally noted were the location of the tracheostomy ring and the thyroid gland.
The area of the supraomohyoid triangle, which matched that of a functional neck dissection in a human, was calculated using these measurements and the Heron formula. The distance from the upper edge of the omohyoid muscle to the upper edge of the sternomastoid muscle was taken to measure side (a). The distance from the upper edge of the sternomastoid muscle till it crossed the omohyoid muscle was employed to measure side (b). The length of the omohyoid muscle from its intersection with the mastoid muscle to its superior insertion was determined for side (c) (Figs. 23):
\(\left(\text{Heron Formula}\right)\mathrm{\acute{A} }=\sqrt{\left(\frac{\left(a+b+c\right)\left(-a+b+c\right)\left(a-b+c\right)\left(a+b-c\right)}{16}\right).}\)
The volume of the submaxillary gland using Solidworks® software was estimated as if it were conformed by an ellipsoid body.
Furthermore, using SPSS®, the means and standard deviation for each measurement we also calculated.
Results
Anatomical measurements of the porcine model
Table 1 lists the anatomical measures, means, and standard deviation for each surgical landmark.
Head and neck surgery training guide on the porcine model
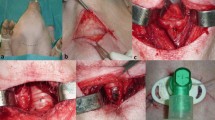
Tracheotomy (Fig. 1 )
All limbs of the porcine model must be fastened and flexed in the supine position, and an appropriate endotracheal tube fixation should be employed (Fig. 11).
Before the training session begins, a number of the animal model peculiarities must be taken into consideration. The sternal-mental distance (X = 15.8 cm, σ = 0.45) is clearly greater than human and may lead to confusion during the first training steps of the trainee. The mental–mento distance is also greater than in humans (x = 11 cm, = 2.3) (Fig. 12).
Platysmal flap elevation, neck dissection and spinal triangle exposure on the live porcine model. Right lateral view. < indicates in all the photos the most cranial plane. a Platysmal flap length, b prelaryngeal (sternohyoid) muscle, c external jugular vein, d cleidooccipital muscle, e brown fat and thymic tissue, f sternomastoid muscle, g omohyoid (cropped in image 4 to allow the jugular, vagus and common carotid veins to be seen), h supraomohyoid triangle: functional neck dissection, i submaxillary gland *. Common carotid artery, “. Vagus nerve, ~ . Internal Jugular vein
Tracheotomy has been shown in the swine model to be an excellent procedure for placing residents who are just beginning to develop these capabilities in a practical scenario [6, 9, 10]. The incision for the tracheostomy should be three finger widths above the upper sternal limit.
If the primary goal of the exercise is the tracheotomy, the dissection is remarkably similar to that done on humans at this point. The prelaryngeal muscles are divided and separated in the midline, with the sternohyoid being the dominant muscle (Figs. 14, 2, 1b).
The thyroid gland can be seen when the prelaryngeal musculature has been separated. We have discovered every time during our dissections that the thyroid gland in pigs is noticeably smaller and lower located in the neck than it is in humans. The thyroid gland has a significantly more friable consistency and is situated in the space between the fifth and sixth tracheal rings. This restricts how thyroid surgery could be trained in the porcine model. Additionally, at this level, the most lateral part of the dissection contains an abundance of thymic tissue, which we can be mistaken for thyroid tissue.
Once the thyroid gland has been ligated, a tracheotomy may be performed at this level that reminds to the one performed in humans. Since the tracheal diameter is comparable to that of a human (x = 1.3 cm, σ = 0.30), standard tracheostomy cannula (Shiley® nº8) or Montandon® tubes are suitable for use (Figs. I2, 31). The most significant difference between this swine model and the human being is that we now find ourselves at a more caudal level, where we can see the tracheal cartilages from the third to the seventh.
Schematic view of the surgical dissection on the porcine model. Tracheotomy, cervical dissection and total laryngectomy. 1. Porcine model, superior view, 2. platysmal flap elevation, 3. removal of thymic tissue and brown fat, prior to initiating cervical dissection, 4. anterior view of the larynx and trachea after separation of the prelaryngeal musculature, 5. view of the posterior esophageal wall after total laryngectomy, 6. detail of the laryngeal and tracheal structure < : anterior view, > : posterior view. a Upper border of the sternum, b lower mandibular arch, c platysmal flap, d prelaryngeal muscles (sternohyoid), e brown fat and thymic tissue, f cleidooccipital muscle, g jugular vein external, h sternomastoid muscle, i omohyoid muscle, j spinal nerve, k submaxillary gland, m vasculonervous bundle (internal carotid artery, internal jugular vein and vagus nerve), n thyroid cartilage, o hyoid bone, p epiglottis, q cricoid cartilage, r trachea, t thyroid gland, s esophageal diverticulum, u posterior esophageal wall, v vocal rudiment
Visceral space and cervical musculature: neck dissection (Figs. 2, 42, 43 and 5)
Spinal triangle detailed on the live porcine model Right lateral view (1) and platysmal flap raising (2). < indicates in all the photos the most cranial plane. a Sternomastoid muscle, b spinal nerve, c internal jugular vein, d supraspinal space, e brown fat and thymic tissue, f prelaryngeal musculature, g submaxillary gland
Total laryngectomy on the live porcine model. Superior view. ^ indicates the most cranial end in all images. a Hyoid bone, b thyrohyoid membrane, c thyroid cartilage, d cricoid cartilage, e trachea, f prelaryngeal musculature (separated to the sides), g vocal rudiment, h direction towards the oropharynx, i direction towards the esophagus, j glottic space, k direction towards the esophageal diverticulum, m cricothyroid membrane, n epiglottis (everted)
After making the U-shaped incision, the platysmal flap will be raised. The pig model's cervical musculature is strong, thick, and occasionally coated with brown fat in its most superficial area. This fact must be considered when raising the flap, and the brown fat must not be damaged (Fig. 21), otherwise we run the danger of losing the anatomical references. If these prior foundations are ignored, this step may be rather difficult.
Once the platysmal flap is raised we will be able to see the complete extension of the prelaryngeal muscle, the cleidooccipital muscle, as well as all of the brown fat and the thymic tissue mixed in with it. The most lateral component of the platysmal flap raising will include a partial section the cleidooccipital muscle. The cleoidooccipital muscle is located in the most caudal and lateral neck region (Fig. 2, d). The sternomastoid muscle (Fig. 2, f) is located medial to the cleidooccipital muscle and is covered with brown fat. When performing the training session, we shall think about the sternomastoid muscle as if it were the sternocleidomastoid muscle in the human being. The internal jugular vein may be seen crossing between the cleidooccipital muscle and the sternomastoid muscle (Fig. 24).
At this point, there are two possible approaches to the dissection:
-
Removing all brown fat and thymic tissue before starting the neck dissection.
-
Combining the visceral tissue with the thymus and the brown fat as part of the neck dissection (Fig. 52)
The swine model contains a lot of thymic tissue, which is a significant component of the visceral region of the neck area. In our experience, the later alternative would be confusing for a less trained surgeon, so we advise against using it.
The omohyoid muscle, digastric muscle, and sternomastoid muscle all serve as the margins of the supraomohyoid triangle, which corresponds to functional neck dissection in humans. Because of the extremely small size of this triangle (x = 7.07 cm2, σ = 3.91), the omohyoid muscle can be cut and combined with the visceral dissected tissue (Fig. 24g). The reason the omohyoid muscle is more horizontal in the porcine model and leaves a smaller supraomohyoid triangle is attributed to a significantly more cranial scapula and a shorter neck in a position of sustained flexion.
It is noteworthy how challenging is to identify the digastric muscle during surgical dissection in pigs. This is because the muscle in question has its origins in the jugular process of the occipital bone and inserts itself in a little fibrous bundle in the body of the mandible. Furthermore, in the porcine model, the digastric muscle has only one belly [11]. Therefore, we chose to draw a straight horizontal line from the insertion of the omohyoid muscle to the insertion of the sternomastoid muscle to estimate the area of the supraomohyoid triangle (Fig. 23).
The huge porcine submandibular gland (x = 6.16 cm3, σ = 0.70) needs to be retracted to reveal the spinal nerve crossing under the sternomastoid muscle clearly (Fig. 51b). Given that the spinal nerve's path and placement are identical to those of a human, it is perfect for replicating the surgical steps in this area.
The internal jugular vein and internal carotid artery, along with the vagus nerve, will be seen once the neck dissection is completed. Internal jugular vein (x = 0.33 cm, σ = 0.049) and common carotid artery (x = 0.38, σ = 0.13) diameters, in this case, are noticeably smaller than those in humans.
Larynx structure (Figs. 3, 44, 46)
The porcine larynx has a tubular shape and is made up of cartilage, perichondrium, connective tissue, and mucus, just like the human larynx. It is divided into the supraglottis, glottis, and subglottis and exhibits the same structural elements as the human glottis: the thyroid cartilage, the cricoid cartilage, and some primitive arytenoid cartilages.
The hyoid bone, which is located above the larynx, has two lesser and two greater horns that are longer than human horns but easily disarticulable (Fig. 31a).
In comparison to humans, the thyroid cartilage is larger. It is shield-shaped, but it lacks the greater horns and the triangular notch (Fig. 31). Furthermore, the thyroid cartilage is connected to the thyrohyoid membrane that is in contact with the hyoid bone (Fig. 31).
The cricoid cartilage is less defined in comparison to humans. Despite this, it has an oval-shaped posterior base ring that articulates with thyroid cartilage in its anterior and superior face via the cricothyroid membrane. The cricoid cartilage continues with the trachea at a lower level and it may be difficult to distinguish its inferior limit from the first tracheal ring.
The epiglottic cartilage is larger than in humans and less rigid. When making an incision through the thyrohyoid membrane to reach the epiglottis, it is common to miss its free border. To evaluate the entire epiglottis, insert a finger into the incision made at the thyrohyoid membrane level and feel for the base of the tongue until reaching this cartilage, which is sometimes flexed and inverted.
We discovered a rudimentary epiglottic cartilage formed by an arytenoid cartilage and some interarytenoid cartilages in the porcine phonatory model, forming what we could call the vocal rudiment (Fig. 3g). The vocal ligament structure is similar to that of humans, but it is much more verticalized.
When the larynx is removed, we can see a diverticulum on the posterior wall of the esophagus (Fig. 34). Although the deficit left by total laryngectomy is slightly greater than in humans, the esophageal stump can be closed.
Discussion
In recent years, there has been a paradigm shift in the treatment of H&N cancers. Radiotherapy, immunotherapy, and chemotherapy have gradually replaced surgery as the first-line treatment option, particularly in cases where a total laryngectomy was previously required [1, 12,13,14,15]. Surgery is now mostly reserved for salvage cases, recurrences, and patients who have already been treated with radiation. The complexity of these procedures, as well as the likelihood of subsequent complications, is unquestionably increased, as are the length of stay, morbidity and mortality, and hospital readmission rates [16, 17].
While hospitals with a high volume of H&N cancer surgery can maintain better surgical standards due to their high specialization, small and medium-sized hospitals, such as those in developing countries, can have a higher rate of complications due to fewer resources [18, 19].
To take the first steps in H&N surgery, it is critical to reconsider instruction and create a simulation model that can provide the resident with an environment as close to realistic operating room conditions as possible.
There are several options when it comes to selecting a model. The rabbit and sheep animal models are among them [20,21,22], but the porcine model is more accurate in simulating human cervical and laryngeal tissues. Other options include using non-organic simulators [4, 23] or virtual reality technology that is still in development [24, 25]. On the other hand, the live porcine model, as opposed to a human or animal cadaver, allows the learner to experience all of the realistic situations that could arise in the operating room such as bleeding, increasing the difficulty and realism of the surgical training [26].
A variety of pig breeds can be recommended for surgical training. Similar initiatives have been documented using the Yorkshire pig [6] or the Sus Scrofa Domestica [27], but the Large White is easier to manage due to its smaller size and lower cost [8]. Because there are so many different varieties, drawing meaningful parallels between size measurements taken from various pig model breeds and those taken from humans is difficult.
Despite the enormous number of procedures documented in the porcine model, there is little literature that defines the anatomy oriented to perform these procedures. Several authors [28, 29], have provided detailed descriptions of the laryngeal anatomy and histology of the porcine model, which have produced excellent results and demonstrated that the endolaryngeal surgery training method is efficient, reproducible, and affordable [30].
The porcine model has also been proposed for training in laryngeal and airway reconstructive surgery [27, 31, 32] as well as reconstructive microsurgery, with promising results [6, 20, 33] Another application for this framework is the investigation of potential therapeutic alternatives, such as laryngeal transplantation [34, 35]. In our previous work, we used the porcine model to demonstrate through a standardized questionnaire [36] the improvement in surgical proficiency of residents, adding construct validity to the model.
The first barrier to access we discovered in our instance, despite reviewing the specialized literature on swine anatomy, was the lack of a bibliography that in-depth described the cervical spaces, the surgical anatomy, and its approach [37, 38]. Regrettably, because pig animals and humans do not receive the same surgical care, we were unable to find any references that could be used to compare the two. This fact was the foundation for the dissection instructions we provide in this paper, which may be useful to future facilities using the porcine model as a training tool in head and neck surgery.
Propst et al. produced a manual in the form of a book in 2014 [7], outlining in great detail how to handle the porcine model before, during, and after performing airway surgery. We have a knowledge deficit in this area since they only covered airway surgeries (tracheotomy and tracheal repair), leaving out the remainder of the head and neck procedures.
The primary limitation of this work is the lack of validation, as there has never been a comprehensive description of these techniques before, nor is there a literature documenting the anatomy of the porcine model. Face and content validity [39,40,41,42] is the most commonly used method for validating surgical simulation models using questionnaires distributed to experts. We believe that comparing the development of surgical skills between residents who received porcine model training and those who did not is a better option. This comparison, however, might be difficult to perform given the scarcity of examples where surgical expertise can be gained, which is the same reason for beginning this type of training.
Despite the limitations mentioned, we think that this guideline contributes to creating and standardizing the porcine model in head and neck surgery training.
Conclusions
This experience has enabled us to assess the current state of training and teaching for head and neck surgery procedures in our hospital, with the goal of optimizing the learning curve for H&N resident surgeons.
The practice of H&N surgery on the porcine model is described in detail, with a focus on tracheotomy, cervical dissection, and laryngectomy procedures. Given the characteristics described, the pig model appears appropriate for teaching in H&N surgery. The primary flaw in our study is the model's lack of validity. We believe that our simulation model is efficient, approachable, and extremely engaging as a tool for the professional development of the otolaryngology resident. The difficulties that lie ahead will be in validating it.
References
Maddox PT, Davies L (2012) Trends in total laryngectomy in the era of organ preservation: a population-based study. Otolaryngol Head Neck Surg 147:85–90. https://doi.org/10.1177/0194599812438170
Chin CJ, Chin CA, Roth K et al (2016) Simulation-based otolaryngology-head and neck surgery boot camp: “how I do it.” J Laryngol Otol 130:284–290. https://doi.org/10.1017/S0022215115003485
Loh CYY, Wang AYL, Tiong VTY et al (2018) Animal models in plastic and reconstructive surgery simulation—a review. J Surg Res 221:232–245. https://doi.org/10.1016/j.jss.2017.08.052
Sutherland LM, Middleton PF, Anthony A et al (2006) Surgical simulation: a systematic review. Ann Surg 243:291–300. https://doi.org/10.1097/01.sla.0000200839.93965.26
Badash I, Burtt K, Solorzano CA, Carey JN (2016) Innovations in surgery simulation: a review of past, current and future techniques. Ann Transl Med. https://doi.org/10.21037/atm.2016.12.24
Deonarain AR, Harrison RV, Gordon KA et al (2019) Live porcine model for surgical training in tracheostomy and open-airway surgery. Laryngoscope. https://doi.org/10.1002/lary.28309
Propst EJ (2014) Airway reconstruction surgical dissection manual. Plural Publishing, San Diego
Alcalá Rueda I, Villacampa Aubá JM, Encinas Vicente A et al (2021) A live porcine model for surgical training in tracheostomy, neck dissection, and total laryngectomy. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-021-06613-y
Netto FACS, Zacharias P, Cipriani RFF et al (2015) A porcine model for teaching surgical cricothyridootomy. Rev Col Bras Cir 42:193–196. https://doi.org/10.1590/0100-69912015003012
Goh CS-L, Joethy J-V, Tan B-K, Wong M (2018) Large animal models for long-segment tracheal reconstruction: a systematic review. J Surg Res 231:140–153. https://doi.org/10.1016/j.jss.2018.05.025
Magalhães HIR, Barcelos JB, Romão FB et al (2021) Comparative study of the digastric and the stylohyoid muscles between wild boars (Sus scrofa scrofa) and domestic swine (Sus scrofa domesticus): revisiting the gross anatomy. Anat Cell Biol 54:202–211. https://doi.org/10.5115/acb.20.301
Takes RP, Strojan P, Silver CE et al (2012) Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck 34:270–281. https://doi.org/10.1002/hed.21613
Orosco RK, Weisman RA, Chang DC, Brumund KT (2013) Total laryngectomy: national and regional case volume trends 1998–2008. Otolaryngol Head Neck Surg 148:243–248. https://doi.org/10.1177/0194599812466645
Mowery A, Light T, Clayburgh D (2018) Long-term trends in head and neck surgery outcomes. Otolaryngol Head Neck Surg 159:1012–1019. https://doi.org/10.1177/0194599818785157
Khaja SF, Hoffman HT, Pagedar NA (2016) Treatment and survival trends in glottic carcinoma in situ and stage I cancer from 1988 to 2012. Ann Otol Rhinol Laryngol 125:311–316. https://doi.org/10.1177/0003489415611908
Gourin CG, Forastiere AA, Sanguineti G et al (2011) Volume-based trends in laryngeal cancer surgery. Laryngoscope 121:77–84. https://doi.org/10.1002/lary.21393
Vimawala S, Topf MC, Savard C et al (2020) Risk factors for unplanned readmission in total laryngectomy patients. Laryngoscope 130:1725–1732. https://doi.org/10.1002/lary.28255
Gourin CG, Stewart CM, Frick KD et al (2019) Association of hospital volume with laryngectomy outcomes in patients with larynx cancer. JAMA Otolaryngol Head Neck Surg 145:62–70. https://doi.org/10.1001/jamaoto.2018.2986
Saraswathula A, Austin JM, Fakhry C et al (2022) Surgeon volume and laryngectomy outcomes. Laryngoscope. https://doi.org/10.1002/lary.30229
Milner TD, Okhovat S, Clement WA et al (2019) A systematic review of simulated laryngotracheal reconstruction animal models. Laryngoscope 129:235–243. https://doi.org/10.1002/lary.27288
Fermi M, Mattioli F, Ghirelli M et al (2021) Open laryngeal surgery training on ex-vivo ovine model: development and dissection experience. Auris Nasus Larynx 48:1150–1156. https://doi.org/10.1016/j.anl.2021.04.001
Fermi M, Chiari F, Mattioli F et al (2022) Surgical training on ex vivo ovine model in otolaryngology head and neck surgery: a comprehensive review. Int J Environ Res Public Health 19:3657. https://doi.org/10.3390/ijerph19063657
Stefanidis D, Sevdalis N, Paige J et al (2015) Simulation in surgery: what’s needed next? Ann Surg 261:846–853. https://doi.org/10.1097/SLA.0000000000000826
Reighard CL, Green K, Powell AR et al (2019) Development of a high fidelity subglottic stenosis simulator for laryngotracheal reconstruction rehearsal using 3D printing. Int J Pediatr Otorhinolaryngol 124:134–138. https://doi.org/10.1016/j.ijporl.2019.05.027
Kovatch KJ, Powell AR, Green K et al (2020) Development and multidisciplinary preliminary validation of a 3-dimensional-printed pediatric airway model for emergency airway front-of-neck access procedures. Anesth Analg 130:445–451. https://doi.org/10.1213/ANE.0000000000003774
Stefanidis D, Yonce TC, Green JM, Coker AP (2013) Cadavers versus pigs: which are better for procedural training of surgery residents outside the OR? Surgery 154:34–37. https://doi.org/10.1016/j.surg.2013.05.001
González-García JA, Chiesa-Estomba CM, Álvarez L et al (2018) Porcine experimental model for perforator flap raising in reconstructive microsurgery. J Surg Res 227:81–87. https://doi.org/10.1016/j.jss.2018.02.025
Papuzinski AC, Garnham PR, Gauna PF et al (2020) Estudio comparativo de laringes humanas y de cerdos: Hacia un modelo de entrenamiento quirúrgico. Rev Otorrinolaringol Cir Cabeza Cuello 80:9–18. https://doi.org/10.4067/S0718-48162020000100009
Garza HH, Vega RF, Zubiaur GF et al (2012) Presentación de un modelo de laringe porcina para el entrenamiento en cirugía laringotraqueal asistida por endoscopia. Otorrinolaringología 57(1):17–24
Ghirelli M, Mattioli F, Federici G et al (2019) Ex vivo porcine larynx model for microlaryngoscopy laryngeal surgery: proposal for a structured surgical training. J Voice. https://doi.org/10.1016/j.jvoice.2019.02.007
González-García JA, Chiesa-Estomba CM, Larruscain E et al (2019) Porcine experimental model for gracilis free flap transfer to the head and neck area with novel donor site description. J Plast Reconstr Aesthet Surg. https://doi.org/10.1016/j.bjps.2019.05.032
Alessa MA, Kwak SH, Lee YW et al (2018) Porcine as a training module for head and neck microvascular reconstruction. J Vis Exp. https://doi.org/10.3791/58104
Mitskavich MT, Rimell FL, Shapiro AM et al (1996) Laryngotracheal reconstruction using microplates in a porcine model with subglottic stenosis. Laryngoscope 106:301–305. https://doi.org/10.1097/00005537-199603000-00011
Birchall MA, Kingham PJ, Murison PJ et al (2011) Laryngeal transplantation in minipigs: vascular, myologic and functional outcomes. Eur Arch Otorhinolaryngol 268:405–414. https://doi.org/10.1007/s00405-010-1355-3
Birchall MA, Ayling SM, Harley R et al (2012) Laryngeal transplantation in minipigs: early immunological outcomes. Clin Exp Immunol 167:556–564. https://doi.org/10.1111/j.1365-2249.2011.04531.x
Hatala R, Cook DA, Brydges R, Hawkins R (2015) Constructing a validity argument for the Objective Structured Assessment of Technical Skills (OSATS): a systematic review of validity evidence. Adv Health Sci Educ Theory Pract 20:1149–1175. https://doi.org/10.1007/s10459-015-9593-1
Bollen PJA, Hansen AK, Alstrup AKO (2010) The laboratory swine. CRC Press/Taylor & Francis, Boca Raton
Swindle MM, Smith AC (2016) Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques, 3rd edn. CRC Press, Boca Raton
AlQahtani A, Albathi A, Castelnuovo P, Alfawwaz F (2021) Cerebrospinal fluid leak repair simulation model: face, content, and construct validation. Am J Rhinol Allergy 35:264–271. https://doi.org/10.1177/1945892420952262
Aucar JA, Groch NR, Troxel SA, Eubanks SW (2005) A review of surgical simulation with attention to validation methodology. Surg Laparosc Endosc Percutan Tech 15:82–89. https://doi.org/10.1097/01.sle.0000160289.01159.0e
Sankaranarayanan G, Parker L, De S et al (2021) Simulation for colorectal surgery. J Laparoendosc Adv Surg Tech A 31:566–569. https://doi.org/10.1089/lap.2021.0096
Enciso S, Díaz-Güemes I, Usón J, Sánchez-Margallo FM (2016) Validation of a model of intensive training in digestive laparoscopic surgery. Cir Esp 94:70–76. https://doi.org/10.1016/j.ciresp.2015.10.005
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest associated with this publication. As corresponding author, I confirm that the manuscript has been read and approved for submission by all authors.
Consent for publication
We declare that this article is original and has not been published in any other journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alcalá Rueda, I., Sánchez Barrueco, Á., Cenjor Español, C. et al. Everything but the squeal: a guide for head and neck surgery training on the live porcine model. Eur Arch Otorhinolaryngol 280, 2927–2936 (2023). https://doi.org/10.1007/s00405-023-07882-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-07882-5