Abstract
Background
The present meta-analysis was updated with randomized controlled trials (RCTs) to revaluate the efficacy and safety of cetuximab vs. cisplatin combined with radiotherapy in patients of head and neck squamous cell carcinoma (HNSCC).
Methods
A meta-analysis containing RCTs that compared the efficacy or toxicity of cetuximab and cisplatin in HNSCC patients was conducted.
Results
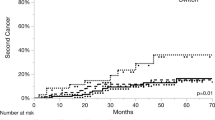
Seven RCTs were included in the final analysis. The patients treated by cetuximab plus radiotherapy showed an inferior overall survival (OS) and locoregional control (LRC) compared to cisplatin plus radiotherapy. The tendency of progression-free survival (PFS) was in agreement with OS and LRC. Subgroup analysis showed that cetuximab had poorer OS relative to cisplatin in the absence of induction chemotherapy. The profile of severe adverse events (SAEs) varied between the two groups, no significant difference in total SAEs was shown for the two arms.
Discussion
Cetuximab combined with radiotherapy shows significantly reduced therapeutic efficacy compared to cisplatin plus radiotherapy in HNSCC patients.






Similar content being viewed by others
Availability of data and materials
All datasets generated for this study are included in the article.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Marur S, Forastiere AA (2016) Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc 91(3):386–396
Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D, Kim H, Silverman C, Raben A, Galloway TJ et al (2014) Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol 32(34):3858–3866
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE et al (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 16(4):1310–1317
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357(20):2040–2048
Chan JA, Blaszkowsky LS, Enzinger PC, Ryan DP, Abrams TA, Zhu AX, Temel JS, Schrag D, Bhargava P, Meyerhardt JA et al (2011) A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann Oncol 22(6):1367–1373
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359(11):1116–1127
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354(6):567–578
Argiris A, Karamouzis MV, Raben D, Ferris RL (2008) Head and neck cancer. Lancet 371(9625):1695–1709
Caudell JJ, Sawrie SM, Spencer SA, Desmond RA, Carroll WR, Peters GE, Nabell LM, Meredith RF, Bonner JA (2008) Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys 71(3):676–681
Magrini SM, Buglione M, Corvo R, Pirtoli L, Paiar F, Ponticelli P, Petrucci A, Bacigalupo A, Crociani M, Lastrucci L et al (2016) Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: a randomized phase II trial. J Clin Oncol 34(5):427–435
Levy A, Blanchard P, Bellefqih S, Brahimi N, Guigay J, Janot F, Temam S, Bourhis J, Deutsch E, Daly-Schveitzer N et al (2014) Concurrent use of cisplatin or cetuximab with definitive radiotherapy for locally advanced head and neck squamous cell carcinomas. Strahlenther Onkol 190(9):823–831
Tang C, Chan C, Jiang W, Murphy JD, von Eyben R, Colevas AD, Pinto H, Lee-Enriquez N, Kong C, Le QT (2015) Concurrent cetuximab versus platinum-based chemoradiation for the definitive treatment of locoregionally advanced head and neck cancer. Head Neck 37(3):386–392
Huang J, Zhang J, Shi C, Liu L, Wei Y (2016) Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC Cancer 16:689
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM, Burtness B et al (2019) Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393(10166):40–50
Hitt R, Mesia R, Grau JJ, Iglesias L, Barco ED, Lozano A, Trufero JM, Giron CG, Martin AL, Hernandez JJC (2016) Randomized phase III trial of induction chemotherapy (ICT) with docetaxel-cisplatin-5fluorouracil (DCF) followed by cisplatin-radiotherapy (CRT) or cetuximab-radiotherapy (CetRT) in patients (pts) with locally advanced unresectable head and neck cancer (LAUHNC). 34(15_suppl):6001
Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O’Toole L et al (2019) Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393(10166):51–60
Gebre-Medhin M, Brun E, Engstrom P, Haugen Cange H, Hammarstedt-Nordenvall L, Reizenstein J, Nyman J, Abel E, Friesland S, Sjodin H et al (2021) ARTSCAN III: a randomized phase III study comparing chemoradiotherapy with cisplatin versus cetuximab in patients with locoregionally advanced head and neck squamous cell cancer. J Clin Oncol 39(1):38–47.
Janoray G, Pointreau Y, Alfonsi M, Sire C, Geoffrois L, de Raucourt D, Bardet E, Calais MH, Garaud P, Calais G (2020) Induction chemotherapy followed by cisplatin or cetuximab concomitant to radiotherapy for laryngeal/hypopharyngeal cancer: long-term results of the TREMPLIN randomised GORTEC trial. Eur J Cancer (Oxford, England: 1990). 133:86–93
Maddalo M, Borghetti P, Tomasini D, Corvo R, Bonomo P, Petrucci A, Paiar F, Lastrucci L, Bonu ML, Greco D et al (2020) Cetuximab and radiation therapy versus cisplatin and radiation therapy for locally advanced head and neck cancer: long-term survival and toxicity outcomes of a randomized phase 2 trial. Int J Radiat Oncol Biol Phys 107(3):469–477
Lefebvre JL, Pointreau Y, Rolland F, Alfonsi M, Baudoux A, Sire C, de Raucourt D, Malard O, Degardin M, Tuchais C et al (2013) Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol 31(7):853–859
Suton P, Skelin M, Rakusic Z, Dokuzovic S, Luksic I (2019) Cisplatin-based chemoradiotherapy vs. cetuximab-based bioradiotherapy for p16-positive oropharyngeal cancer: an updated meta-analysis including trials RTOG 1016 and De-ESCALaTE. Eur Arch Otorhinolaryngol 276(5):1275–1281
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363(1):24–35
Richards L (2010) Human papillomavirus-a powerful predictor of survival in patients with oropharyngeal cancer. Nat Rev Clin Oncol 7(9):481
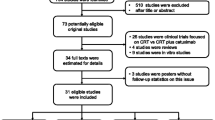
Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 6(7):e1000097
Furlan AD, Pennick V, Bombardier C, van Tulder M, Editorial Board CBRG (2009) 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 34(18):1929–1941
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17(24):2815–2834
Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codeca C, Nole F, Verri E, Orecchia R, Morelli F et al (2017) Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol 28(9):2206–2212
Taberna M, Oliva M, Mesia R (2019) Cetuximab-containing combinations in locally advanced and recurrent or metastatic head and neck squamous cell carcinoma. Front Oncol 9:383
Riaz N, Sherman E, Koutcher L, Shapiro L, Katabi N, Zhang Z, Shi W, Fury M, Wong R, Wolden S et al (2016) Concurrent chemoradiotherapy with cisplatin versus cetuximab for squamous cell carcinoma of the head and neck. Am J Clin Oncol 39(1):27–31
Ley J, Mehan P, Wildes TM, Thorstad W, Gay HA, Michel L, Nussenbaum B, Trinkaus K, Adkins D (2013) Cisplatin versus cetuximab given concurrently with definitive radiation therapy for locally advanced head and neck squamous cell carcinoma. Oncology 85(5):290–296
Hu MH, Wang LW, Lu HJ, Chu PY, Tai SK, Lee TL, Chen MH, Yang MH, Chang PM (2014) Cisplatin-based chemotherapy versus cetuximab in concurrent chemoradiotherapy for locally advanced head and neck cancer treatment. Biomed Res Int 2014:904341
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11(1):21–28
Rosenthal DI, Harari PM, Giralt J, Bell D, Raben D, Liu J, Schulten J, Ang KK, Bonner JA (2016) Association of human papillomavirus and p16 status with outcomes in the IMCL-9815 phase III registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without cetuximab. J Clin Oncol 34(12):1300–1308
Beckham TH, Barney C, Healy E, Wolfe AR, Branstetter A, Yaney A, Riaz N, McBride SM, Tsai CJ, Kang J et al (2020) Platinum-based regimens versus cetuximab in definitive chemoradiation for human papillomavirus-unrelated head and neck cancer. Int J Cancer 147(1):107–115
Safran H, Suntharalingam M, Dipetrillo T, Ng T, Doyle LA, Krasna M, Plette A, Evans D, Wanebo H, Akerman P et al (2008) Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys 70(2):391–395
Pryor DI, Porceddu SV, Burmeister BH, Guminski A, Thomson DB, Shepherdson K, Poulsen M (2009) Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother Oncol 90(2):172–176
Bar-Ad V, Zhang QE, Harari PM, Axelrod R, Rosenthal DI, Trotti A, Jones CU, Garden AS, Song G, Foote RL et al (2016) Correlation between the severity of cetuximab-induced skin rash and clinical outcome for head and neck cancer patients: the RTOG experience. Int J Radiat Oncol Biol Phys 95(5):1346–1354
Weycker D, Li X, Tzivelekis S, Atwood M, Garcia J, Li Y, Reiner M, Lyman GH (2017) Burden of chemotherapy-induced febrile neutropenia hospitalizations in US clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Supportive Care Cancer 25(2):439–447
Sheth S, Mukherjea D, Rybak LP, Ramkumar V (2017) Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front Cell Neurosci 11:338
Acknowledgements
We would like to thank Bashir Kaspo (University of Michigan) for his guidance on the language of this article.
Funding
This study was partially supported by the National Natural Science Foundation of China (no. 81202962).
Author information
Authors and Affiliations
Contributions
Study conception and design, data interpretation, and manuscript writing and reviewing: YL, YQ, and FL. Literature review: YL and CY. Data extraction: CY and YQ. Statistical analysis: YG and YL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval and Consent to participate
Not applicable.
Consent for publication
All authors of the manuscript have read and approved the manuscript, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
SupplementaryFig. 1 Meta-analysis results of 3-year LRC without induction chemotherapy.
*The long-term results of Lefebvre’s studyreported by Janoray et al. in 2020 were also included in the final analysis (PDF 504 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Yang, C., Gan, Y. et al. Radiotherapy plus cetuximab or cisplatin in head and neck squamous cell carcinoma: an updated systematic review and meta-analysis of randomized controlled trials. Eur Arch Otorhinolaryngol 280, 11–22 (2023). https://doi.org/10.1007/s00405-022-07572-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-022-07572-8