Abstract
Objectives
The questionnaire of olfactory disorder-negative statements (QOD-NS) is a valid and reliable instrument for assessing the olfactory-specific quality of life. This study aimed to explore the association between the QOD-NS and objective olfactory metrics (including objective olfactory cleft assessment) and then evaluate the predictive significance of the QOD-NS for olfactory loss in Chinese patients with chronic rhinosinusitis (CRS).
Methods
A total of 70 patients with CRS were enrolled in the study. Olfaction was assessed with Sniffin’ Sticks. The olfactory cleft was assessed by the sinus CT scan and nasal endoscopy (the Lund–Mackay olfactory cleft scale, LM-OC and olfactory cleft endoscopy scale, OCES). The QOD-NS and its short version were utilized to assess the patient-reported olfaction. The predictors associated with olfactory loss were analyzed by the logistic regression analysis. The optimal cutoff points of the predictors were determined by the receiver-operating characteristic curves and the Youden index.
Results
The TDI score in patients with CRS significantly correlated with the QOD-NS (r = − 0.755, P < 0.001), OCES (r = − 0.520, P < 0.001), LM-OC (r = − 0.615, P < 0.001). After adjusting for patient demographics and comorbidities, QOD-NS was significantly associated with olfactory dysfunction [odds ratio (OR) = 1.243; P = 0.001] and anosmia in patients with CRS (OR = 1.838; P = 0.006). Furthermore, the QOD-NS significantly correlated with the LM-OC (r = 0.610, P < 0.001), and the OCES (r = 0.464, P < 0.001) in patients with CRS. The QOD-NS had the highest predictive value for olfactory dysfunction (optimal cutoff = 10.5; Youden index = 0.635; area under the curve = 0.861) and anosmia (optimal cutoff = 20.5; Youden index = 0.790; area under the curve = 0.928) in patients with CRS.
Conclusion
The QOD-NS showed high validity and correlated well with objective olfactory metrics and olfactory cleft assessment in patients with CRS. The QOD-NS was a reliable predictor for olfactory dysfunction and anosmia in patients with CRS, which may aid in the fast screening of olfactory loss in the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is a chronic inflammatory of the upper airway and is one of the most frequent causes of olfactory dysfunction, accounting for 20–30% of all etiologies [1, 2]. Olfactory dysfunction is a key symptom of CRS and affects approximately 67–78% of patients with CRS [3]. Previous studies showed impaired olfaction was highly associated with increased medication use and poor quality of life (QOL) [4, 5]. A large proportion of patients experiencing olfactory dysfunction may have less interest in food and appear to increase the risk for clinical depression [6, 7]. Given the severity of olfactory dysfunction, olfaction should be routinely evaluated in patients with CRS.
Psychophysical olfactory tests and subjective testing have been developed to evaluate olfaction [1]. Screening tools based on psychophysical olfactory tests are used extensively in clinical practice and research, such as the “Sniffin’ Sticks” 12-item screening test and the Cross-Cultural Smell Identification Test (CCSIT) [8,9,10]. The advantage of these tests is their short duration. However, test results are influenced by the familiarity with odors and depend on the cognition and language abilities of subjects. In addition, considering the learning effects of repeated testing and environmental demands, their clinical applicability is somewhat limited. The questionnaire of olfactory disorders-negative statements (QOD-NS) composed of 17 self-reporting items is an important instrument for assessing patient-reported olfactory-specific QOL [11, 12]. The QOD-NS, which was modified from the questionnaire of olfactory disorders (QOD), has been confirmed for more efficiency, less burden, higher completion and an increase in response rates [11]. Specifically, it evaluates olfactory function focused on social, anxiety, annoyance, and eating-related subdomain. The QOD-NS has been previously validated and shown a good correlation with olfactory loss in CRS [5, 13]. Previous studies have demonstrated that it is possible to measure treatment effects in patients with CRS-associated olfactory dysfunction by the QOD-NS [14, 15]. However, there have been few studies focused on the predictive role of the QOD-NS for olfactory dysfunction in patients with CRS. Furthermore, patients with CRS have shown unique changes within the olfactory cleft which could be objectively measured by sinus CT scan and nasal endoscopy, which have been identified to be associated with the degree of olfactory dysfunction [16, 17]. The association between the QOD-NS and objective olfactory cleft assessment in patients with CRS has not been explored.
Based on these findings, we hypothesized that the QOD-NS may be associated with the olfactory cleft and that analysis of QOD-NS may aid in screening which patients are at risk for olfactory dysfunction. The purpose of this study was to explore the association between the QOD-NS and objective olfactory metrics (including objective olfactory cleft assessment) and then to evaluate the predictive significance of the QOD-NS for olfactory loss in Chinese patients with CRS.
Methods
Study design and patients
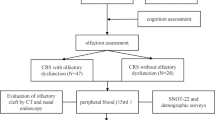
This study was approved by the Ethics Committee of Beijing Anzhen Hospital. Adult patients with a diagnosis of CRS as defined by the European Position Paper on Rhinosinusitis and Nasal Polyps 2020 (EPOS20) were enrolled at the Department of Otolaryngology, Smell and Taste Center, Beijing Anzhen Hospital, Capital Medical University from December 2020 to October 2021 (Fig. 1) [18]. All the patients who provided written informed consent before participation were aware of the aim, method, and clinical implications of the study. Inclusion criteria included patients who underwent endoscopy (the olfactory cleft endoscopy scale, OCES), had a sinus computed tomography scan (Lund–Mackay olfactory cleft score, LM-OC), and olfactory psychophysical test (Sniffin’ Stick test) and patient-reported olfactory-specific quality of life (QOL) questionnaires. Demographic characteristics were further collected. Medical comorbidities, such as asthma, depression, allergic rhinitis, and aspirin-exacerbated respiratory disease (AERD), were specifically collected from the patients. Exclusion criteria included fungal sinusitis, cystic fibrosis, any malignancy, antibiotic or oral corticosteroids medications within the last month, a history of immunodeficiency, prior head trauma, history of neuropsychiatric disorders (i.e., dementia, Alzheimer’s disease, or Parkinson’s disease). Additional exclusion criteria included patients who did not complete all the questionnaires or underwent sinonasal or olfactory examinations.
Psychophysical olfactory tests
Olfactory performance was assessed by Sniffin’ Sticks tests (Burghardt, Wedel, Germany), which were consisted of three subtests: olfactory threshold (OT), odor discrimination (OD), odor identification (OI) [19]. Due to its validated utility and feasibility in a clinic, the “Sniffin’ Sticks” test has been extensively used to evaluate olfactory function since the first publication [20,21,22]. In addition, it has been evaluated in healthy Chinese adults and patients with varied causes of olfactory dysfunction in our previous studies [23,24,25,26,27]. Sniffin’ Sticks is suitable for clinical practice in the Chinese population to differentiate normosmia (normal olfactory function), hyposmia (impaired olfactory function), or anosmia (absent olfactory function) [28]. Felt-tip pens with various odors were used to test patients in a ventilated room. The OT test was performed in a single-staircase method, starting at the lowest dilution of n-butanol and moving to the maximum odor intensity. The OD test used triplet pens which contained two same odorants and one difference. 16 smell pens dispensing different odorants were used in the OI test. Discrimination and identification were scored from 0–16, and threshold was scored from 1–16. Results were aggregated to a composite score of threshold, discrimination, and identification (TDI) which ranged from 1 to 48, with higher scores indicating superior olfactory performance. Values of 15 or less indicated anosmia, values between 16 and 30.75 indicated hyposmia, and values over 30.75 indicated normosmia [29]. In this study, we defined olfactory loss as TDI < 30.75 which included patients with olfactory dysfunction and anosmia.
Patient-reported olfactory assessment
The patient-reported olfactory assessment was performed using the questionnaire of olfactory disorders-negative statements (QOD-NS) and a short version of QOD-NS (sQOD-NS) [11, 30]. As one of the most widely used questionnaires, the QOD-NS had multi-language versions available including Mandarin, English, and Korean [31,32,33]. It was used to evaluate the impact of olfactory dysfunction on QOL among patients with varied causes of olfactory dysfunction [5, 34]. This QOD-NS questionnaire consists of 17 negative statements about the degree to which patients suffered from olfactory impairment. Patients can agree, partly agree, partly disagree, or disagree in each statement which ranges from 0 to 3. A total score of 0–51 is calculated with higher scores reflecting worse olfactory-specific QOL. Mattos et al. developed an sQOD-NS questionnaire which had included large samples for reliability and validation [30]. For the sQOD-NS questionnaire composed of 7 items, the total scores range from 0 to 21.
Objective olfactory cleft assessment measured by sinus CT and nasal endoscopy
In this study, the mucosal conditions around the olfactory cleft were evaluated with sinus CT and nasal endoscopy. The sinus CT scans were scored using the Lund–Mackay olfactory cleft scale (LM-OC) [35, 36]. This scale is currently considered the radiologic staging system of the olfactory cleft lesions due to its high intra- and interobserver agreement and responsiveness [37, 38]. The olfactory cleft is a 3-dimensional space and the borders in CT are defined as follows: anterior (anterior attachment of the middle turbinate); posterior (anterior to the face of the sphenoid sinus); medial (nasal septum); and lateral borders (attachment of the middle and superior turbinate). The superior border is the cribriform plate and the inferior border is the inferior portion of the middle turbinate. The anterior end of the superior turbinate divides the olfactory cleft into anterior and posterior. The percentage of each olfactory cleft that is opacified is graded from 0 to 2, with 0 (no abnormality), 1 (partial opacification), and 2 (total opacification). An overall score of the olfactory cleft on both sides ranging from 0 to 8 is then calculated and higher scores indicated a greater burden of disease on imaging. Olfactory cleft was also evaluated for all patients using nasal endoscopy and graded using the olfactory cleft endoscopy scale (OCES) [39]. The previous study demonstrated its strong validity and provided a high correlation between the degree of olfactory cleft lesions and olfaction [40]. The degree of discharge, nasal polyposis, edema, crusting, and scarring scores from 0 to 2 points for each measure. The sum of the olfactory cleft score on both sides is recorded for final results that ranged from 0 to 20 points. Higher scores indicate increased disease severity of the olfactory cleft. It was scored by experienced rhinologists who were blinded to the diagnosis of the patients.
Sample size calculation
We determined the sample size using PASS 15.0.5 software. This study was a case–control study. The olfactory dysfunction group was the experimental group, and the normal olfactory group was the control group. The score of the QOD-NS was the main observation indicator in the present study. According to a recent study [11], it was estimated that the average QOD-NS score of the experimental group was 19.35 ± 9.16 and the score of the control group was 8.38 ± 6.86. Set α = 0.05 (two-sided), β = 0.20, R = 0.25, 20% non-response rate, the project sample size of the experimental group and the control group was at least 42 (N1) and 12 (N2), respectively.
Statistical analysis
Statistical analysis was completed using SPSS software (version 25.0, IBM Corporation, New York, NY, USA). According to the data distribution, quantitative variables were expressed as mean ± standard deviation (SD) or median (with range or interquartile range, IQR) and qualitative variables were expressed as percentages. All parameters were tested for normal distributions using the one-sample Kolmogorov–Smirnov test. The independent-sample t test was used for continuous variables that followed a normal distribution and Mann–Whitney U test was used for the nonparametric data distribution. The Chi-square (χ2) test was used to compare categorical data. The correlation among the Sniffin’ Stick, QOD-NS, sQOD-NS, OCES and LM-OC was evaluated by Spearman’s rank correlation coefficients (R). To identify independent variables associated with olfactory loss, a binary logistic regression analysis was performed. Receiver-operating characteristic (ROC) curves were used to assess the predictive value of clinical parameters related to olfactory function by the areas under the curve (AUC) determinations and the exact cutoff points which were calculated by Youden’s index. A P value of less than 0.05 was considered statistically significant.
Results
Demographics of the enrolled patients
A total of 70 patients diagnosed with CRS were enrolled in the study. All the demographic characteristics of patients are presented in Table 1. 65.7% of the study cohort was male and the group had an average age of approximately 43.6 years (SD = 12.9). 36 patients (51.4%) presented with chronic rhinosinusitis with nasal polyps (CRSwNP) and 8 patients (11.4%) had a history of sinus surgery.
Olfactory-specific parameters in CRS patients with olfactory dysfunction or anosmia
There were 44 patients with CRS with olfactory dysfunction (62.86%) and 26 patients with CRS without olfactory dysfunction (37.14%) (Table 2). Patients with CRS with olfactory dysfunction had significantly lower TDI, OT, OD, and OI scores than patients with CRS without olfactory dysfunction (all P < 0.001). Patients with CRS with olfactory dysfunction had significantly higher olfactory-specific scores (including the QOD-NS and sQOD-NS) than patients with CRS without olfactory dysfunction (all P < 0.001). Compared with CRS without olfactory dysfunction, LM-OC scores (P = 0.042) were significantly higher in patients with CRS with olfactory dysfunction. There was no significant difference between the two groups regarding the OCES score (P = 0.145).
There were 16 patients with CRS with anosmia (22.86%) and 54 patients with CRS without anosmia (77.14%) (Table 3). Patients with CRS with anosmia had significantly lower TDI, OT, OD, and OI scores than patients with CRS without anosmia (all P < 0.001). Patients with CRS with anosmia had significantly higher olfactory-specific scores (including the QOD-NS, and sQOD-NS), OCES, and LM-OC scores than patients with CRS without anosmia (all P < 0.001).
Correlation between clinical parameters and olfactory function
Across the overall CRS cohort, spearman’s rank correlations between the olfactory-specific QOL, OCES, LM-OC, and Sniffin’ Stick are described in Table 4. The majority of parameters had a negative relationship with olfaction, which indicated that the higher the specific metrics score the worse the olfactory function. Strong correlations were found in the olfactory-specific QOL as measured by the QOD-NS (r = − 0.755, P < 0.001) and sQOD-NS (r = − 0.635, P < 0.001), and it also correlated more significantly than OCES (r = − 0.520, P < 0.001), LM-OC (r = − 0.615, P < 0.001). In CRS patients, the olfactory-specific QOL was significantly correlated with Sniffin’ Stick threshold (OT), discrimination (OD), and identification domain scores (OI). All subdomains had negative correlations with the olfactory cleft score including OCES and LM-OC. In addition, the QOD-NS showed positive correlations with the LM-OC (r = 0.610, P < 0.001) and the OCES (r = 0.464, P < 0.001) in patients with CRS (Fig. 2).
Binary logistic regression analysis for patients in CRS with olfactory dysfunction or anosmia
After adjusting for age, sex, nasal polyps, asthma, smoking status, prior sinus surgery, the QOD-NS was significantly associated with olfactory dysfunction (odds ratio (OR) = 1.243; 95% confidence interval (CI) = 1.092–1.416; P = 0.001] and anosmia (OR = 1.838; 95% CI = 1.186–2.849; P = 0.006) in patients with CRS.
Utility of clinical parameters for patients with olfactory dysfunction and anosmia
The ROC curve analyses were used to summarize the discriminatory capability of clinical parameters for olfactory dysfunction and anosmia (Table 5; Fig. 3a). The area under the ROC curve (AUC) is shown in Table 5. The QOD-NS had a higher accuracy as a discriminatory instrument for patients in CRS with olfactory dysfunction (AUC = 0.861) than the sQOD-NS (AUC = 0.762). Both LM-OC and OCES had low discriminatory capability for CRS with olfactory dysfunction (LM-OC: AUC = 0.637, P = 0.056; OCES: AUC = 0.604, P = 0.150). The maximum Youden’s index was used to determine the optimal cutoff point and demonstrated diagnostic predictive ability for each parameter. Table 5 shows that the QOD-NS with a cutoff point greater than 10.5 indicated a higher predictive accuracy (specificity of 88.5%, sensitivity of 75.0%, and Youden index of 0.635) and the sQOD-NS with a cutoff point greater than 3.5 indicated a higher predictive accuracy (specificity of 90.1%, sensitivity of 57.6%, and Youden index of 0.477).
Compared to other olfactory metrics, the QOD-NS exhibited the highest discriminatory capability for CRS with anosmia (AUC = 0.928). Surprisingly, all clinical parameters showed good AUC values and these data also had statistical significance (Table 6; Fig. 3b). The diagnostic values of each parameter at the cutoff value are shown in Table 6. The optimal cutoff value of the QOD-NS of 20.5 or more yielded a higher predictive accuracy (specificity of 85.2%, sensitivity of 93.8%, and Youden index of 0.790) and the optimal cutoff value of the sQOD-NS of 7.5 or more yielded a higher predictive accuracy (specificity of 77.8%, sensitivity of 87.5%, and Youden index of 0.653). In addition, LM-OC and OCES also exhibited comparable accuracy as the predictor of anosmia (LM-OC: AUC = 0.860, P < 0.001; OCES: AUC = 0.774, P = 0.001). When the LM-OC score was 3.5 or higher (Youden index of 0.646), the sensitivity was 81.3%, and the specificity was 83.3%, respectively. For the OCES, the cutoff point was 6.5 (specificity of 79.6%, sensitivity of 68.8%, and Youden index of 0.484).
Discussion
Understanding the olfactory outcome in patients with CRS requires a comprehensive and accurate olfactory evaluation. The QOD-NS is an efficient instrument designed to provide subjective information concerning the olfactory-specific QOL [11, 12]. Previous studies have demonstrated that the QOD-NS is a reliable method to evaluate olfactory dysfunction and can be helpful for post-treatment follow-up [5, 41]. However, the diagnostic value of the QOD-NS in patients with CRS with olfactory loss is not clear and there is no study focused on the association between the QOD-NS and objective olfactory cleft assessment.
Our study showed that the QOD-NS was significantly higher in patients with CRS with olfactory dysfunction or anosmia. The olfactory-related quality of life in patients with CRS with olfactory dysfunction or anosmia was severe impaired. Furthermore, we found that the QOD-NS had a better correlation with TDI than other parameters including the sQOD-NS, OCES, and LM-OC. Among the TDI subdomain, the QOD-NS had relatively strong correlations with odor threshold and discrimination. A cohort study of 1226 patients with olfactory dysfunction of various causes showed that odor threshold appears to be preferentially decreased than odor discrimination and identification in CRS [42]. A position paper on olfactory dysfunction pointed out that patients with olfactory dysfunction due to sinonasal disease were particularly impaired in the threshold [1].On the other hand, odor discrimination best reflects the olfactory change in patients with CRS after treatment [43]. Taken together, it suggests that the QOD-NS could be a potential instrument to identify olfactory dysfunction or anosmia in patients with CRS. In addition, the binary logistic regression analysis demonstrated that the QOD-NS was an independent predictor for patients with CRS with olfactory dysfunction or anosmia.
Both LM-OC and OCES had a significant negative correlation with olfaction measured by Sniffin’ Sticks tests. Previous studies showed that the olfactory cleft evaluation provided important information regarding the olfactory function and the olfactory cleft area was closely related to the onset of CRS with olfactory loss [40, 44]. Physical obstruction in the olfactory cleft can cause conductive olfactory loss which is the primary mechanism of CRS with olfactory dysfunction [45]. We further showed that objective olfactory metrics about the olfactory cleft including LM-OC and OCES had a positive correlation with the QOD-NS score in patients with CRS with olfactory dysfunction or anosmia. The high correlation with objective olfactory parameters focusing on the olfactory cleft suggests strong validity of the QOD-NS and further promotes the clinical utility of the QOD-NS in patients with CRS.
This study found that both the QOD-NS (AUC = 0.861) and sQOD-NS (AUC = 0.762) had a higher accuracy than other clinical parameters to screen olfactory dysfunction in patients with CRS. These data of the QOD-NS (AUC = 0.923) and sQOD-NS (AUC = 0.881) were more significant in anosmia. When the Youden index was at the highest point of 0.790, the optimal cutoff point of > 10.5 QOD-NS indicated a specificity of 85.2% and a sensitivity of 93.8% for olfactory dysfunction. We found that the QOD-NS was more sensitive and specific than the sQOD-NS to identify olfactory dysfunction. It would be an auxiliary tool to classify olfactory dysfunction. The optimal cutoff point of > 20.5 for the QOD-NS was established in our study which would be helpful to discriminate anosmia in CRS. In addition, we addressed the optimal cutoff point of > 3.5 and 7.5 in the sQOD-NS that were used for discriminating olfactory dysfunction and anosmia, respectively. This establishment of the cutoff point is helpful for the classification of olfaction and can be used for screening in clinical practice. We also found the olfactory cleft score including the LM-OC score (AUC = 0.860) and OCES score (AUC = 0.774) had a high accuracy of screening. The optimal cutoff point of LM-OC and OCES could be 3.5 and 6.5, which was determined by the highest Youden index of 0.646 and 0.484, respectively. The higher LM-OC and OCES score in those who got CRS might be expected to reflect severe olfactory function.
Validate tools have been developed for olfactory screening, such as the CCSIT or the “Sniffin’ Sticks” 12-item screening test [8,9,10]. These screening tests based on orthonasal olfactory function are meant to differentiate between olfactory loss and normosmia [46]. Their advantage is also the short duration. Some disadvantages are that test results are influenced by the familiarity with odors and depend on the cognition and language abilities of subjects. If subjects are unable to recognize odors because of local culture, then the reliability of the test decreases [47]. Clinical application is limited by the learning effects of repeated testing and environmental demands. In addition, odor preservation requires a shelf-life, whether using an olfactory felt-tip or paper with microencapsulated odors [8,9,10]. The QOD-NS has the characteristics of rapid screening and is not limited by the above conditions. Moreover, the QOD focuses on the effect of olfaction on QOL. Our study confirms its high validity and ability to distinguish the degree of olfactory dysfunction. However, a previous study showed that some patients had poor self-rating abilities [48]. This may be due to the patients’ neglect of olfaction and self-psychological effects. To reduce the self-reported errors and improve the comprehensiveness of evaluation, we suggest an extensive psychophysical test of olfactory function can be further performed after the QOD-NS.
There are several limitations to our study. First, this is a cross-sectional study and the results should be validated in further large and longitudinal studies examining olfactory dysfunction in CRS. Second, the scope of this study is limited to Chinese patients. Finally, the sample size is relatively small and subjective olfactory function may be influenced by psychological and emotional factors. Greater insights from a large sample size need to be further validated in future studies. Future studies could also focus on QOD-NS in olfactory dysfunction resulting from other etiologies, such as upper respiratory tract infection or traumatic injury. Given its good validity and discriminant validity, we speculate that the use of the QOD-NS may facilitate rapid screening of a large number of COVID-19 patients with olfactory dysfunction.
Conclusion
Our study showed that the QOD-NS in patients with CRS had high validity and a strong association with objective olfactory metrics and olfactory cleft assessment. The QOD-NS was an efficient tool to screen for olfactory dysfunction in patients with CRS.
References
Hummel T, Whitcroft KL, Andrews P et al (2016) Position paper on olfactory dysfunction. Rhinology 56(1):1–30. https://doi.org/10.4193/Rhin16.248
Whitcroft KL, Hummel T (2019) Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg 145(9):846–853. https://doi.org/10.1001/jamaoto.2019.1728
Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM (2017) The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope 127(2):309–320. https://doi.org/10.1002/lary.26316
Katotomichelakis M, Simopoulos E, Zhang N, Tripsianis G, Danielides G, Livaditis M, Bachert C, Danielides V (2013) Olfactory dysfunction and asthma as risk factors for poor quality of life in upper airway diseases. Am J Rhinol Allergy 27(4):293–298. https://doi.org/10.2500/ajra.2013.27.3903
Mattos JL, Schlosser RJ, Storck KA, Soler ZM (2017) Understanding the relationship between olfactory-specific quality of life, objective olfactory loss, and patient factors in chronic rhinosinusitis. Int Forum Allergy Rhinol 7(7):734–740. https://doi.org/10.1002/alr.21940
Smeets MAM, Veldhuizen MG, Galle S, Gouweloos J, de Haan AJA, Vernooij J, Visscher F, Kroeze JHA (2009) Sense of smell disorder and health-related quality of life. Rehabil Psychol 54(4):404–412. https://doi.org/10.1037/a0017502
Neuland C, Bitter T, Marschner H, Gudziol H, Guntinas-Lichius O (2011) Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope 121(4):867–872. https://doi.org/10.1002/lary.21387
Doty RL, Marcus A, Lee WW (1996) Development of the 12-item cross-cultural smell identification test (CC-SIT). Laryngoscope 106(3 Pt 1):353–356. https://doi.org/10.1097/00005537-199603000-00021
Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S (1996) “Sniffin’ sticks”: screening of olfactory performance. Rhinology 34(4):222–226
Mueller C, Renner B (2006) A new procedure for the short screening of olfactory function using five items from the “Sniffin’ Sticks” identification test kit. Am J Rhinol 20(1):113–116
Simopoulos E, Katotomichelakis M, Gouveris H, Tripsianis G, Livaditis M, Danielides V (2012) Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. Laryngoscope 122(7):1450–1454. https://doi.org/10.1002/lary.23349
Mattos JL, Schlosser RJ, DeConde AS, Hyer M, Mace JC, Smith TL, Soler ZM (2018) Factor analysis of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 8(7):777–782. https://doi.org/10.1002/alr.22112
Rudmik L, Hopkins C, Peters A, Smith TL, Schlosser RJ, Soler ZM (2015) Patient-reported outcome measures for adult chronic rhinosinusitis: A systematic review and quality assessment. J Allergy Clin Immunol 136(6):1532-1540.e2. https://doi.org/10.1016/j.jaci.2015.10.012
Soler ZM, Smith TL, Alt JA, Ramakrishnan VR, Mace JC, Schlosser RJ (2016) Olfactory-specific quality of life outcomes after endoscopic sinus surgery. Int Forum Allergy Rhinol 6(4):407–413. https://doi.org/10.1002/alr.21679
Thomas AJ, Mace JC, Ramakrishnan VR, Alt JA, Mattos JL, Schlosser RJ, Soler ZM, Smith TL (2020) Quality-of-life and olfaction changes observed with short-term medical management of chronic rhinosinusitis. Int Forum Allergy Rhinol 10(5):656–664. https://doi.org/10.1002/alr.22532
Lund VJ, Mackay IS (1993) Staging in rhinosinusitus. Rhinology 31(4):183–184
Chang H, Lee HJ, Mo JH, Lee CH, Kim JW (2009) Clinical implication of the olfactory cleft in patients with chronic rhinosinusitis and olfactory loss. Arch Otolaryngol Head Neck Surg 135(10):988–992. https://doi.org/10.1001/archoto.2009.140
Fokkens WJ, Lund VJ, Hopkins C et al (2020) European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 58(Suppl S29):1–464. https://doi.org/10.4193/Rhin20.600
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) “Sniffin” sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22(1):39–52. https://doi.org/10.1093/chemse/22.1.39
Haehner A, Mayer AM, Landis BN et al (2009) High test-retest reliability of the extended version of the “Sniffin’ Sticks” test. Chem Senses 34(8):705–711. https://doi.org/10.1093/chemse/bjp057
Neumann C, Tsioulos K, Merkonidis C, Salam M, Clark A, Philpott C (2012) Validation study of the “Sniffin’ Sticks” olfactory test in a British population: a preliminary communication. Clin Otolaryngol 37(1):23–27. https://doi.org/10.1111/j.1749-4486.2012.02431.x
van Spronsen E, Ebbens F, Fokkens W (2013) Olfactory function in healthy children: normative data for odor identification. Am J Rhinol Allergy 27(3):197–201. https://doi.org/10.2500/ajra.2013.27.3865
Su B, Bleier B, Wei Y, Wu D (2021) Clinical implications of psychophysical olfactory testing: assessment, diagnosis, and treatment outcome. Front Neurosci 15:646956. https://doi.org/10.3389/fnins.2021.646956
Su B, Wu D, Wei Y (2021) Development of Chinese odor identification test. Ann Transl Med 9(6):499. https://doi.org/10.21037/atm-21-913
Wu D, Li Y, Bleier BS, Wei Y (2020) Superior turbinate eosino-philia predicts olfactory decline in patients with chronic rhinosi-nusitis. Ann Allergy Asthma Immunol 125(3):304-310.e301. https://doi.org/10.1016/j.anai.2020.04.027
Huang T, Wei Y, Wu D (2021) Effects of olfactory training on posttraumatic olfactory dysfunction: a systematic review and meta-analysis. Int Forum Allergy Rhinol 11(7):1102–1112. https://doi.org/10.1002/alr.22758
Liu Y, Fang F, Zhan X, Yao L, Wei Y (2020) The impact of obstructive apnea sleep syndrome on chemical function. Sleep Breath 24(4):1549–1555. https://doi.org/10.1007/s11325-020-02022-3
Yang L, Wei YX, Ren YY, Yu D, Sun YX, Yang BB (2013) Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 48(9):741–745
Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T (2019) Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol 276(3):719–728. https://doi.org/10.1007/s00405-018-5248-1
Mattos JL, Edwards C, Schlosser RJ et al (2019) A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 9(10):1144–1150. https://doi.org/10.1002/alr.22392
Yang D, Wang J, Ni D, Liu J, Wang X (2016) Reliability and validity of the Chinese version of the questionnaire of olfactory disorders (QOD) when used with patients having olfactory dysfunction. Eur Arch Otorhinolaryngol 273(10):3255–3261. https://doi.org/10.1007/s00405-015-3869-1
Langstaff L, Pradhan N, Clark A et al (2019) Validation of the olfactory disorders questionnaire for English-speaking patients with olfactory disorders. Clin Otolaryngol 44(5):715–728. https://doi.org/10.1111/coa.13351
Choi WR, Jeong HY, Kim JH (2018) Reliability and validity of the Korean version of the Questionnaire of Olfactory Disorders. Int Forum Allergy Rhinol 8(12):1481–1485. https://doi.org/10.1002/alr.22186
Ahmedy F, Mazlan M, Danaee M, Abu Bakar MZ (2020) Post-traumatic brain injury olfactory dysfunction: factors influencing quality of life. Eur Arch Otorhinolaryngol 277(5):1343–1351. https://doi.org/10.1007/s00405-020-05823-0
Oluwole M, Russell N, Tan L, Gardiner Q, White P (1996) A comparison of computerized tomographic staging systems in chronic sinusitis. Clin Otolaryngol Allied Sci 21(1):91–95
Metson R, Gliklich RE, Stankiewicz JA et al (1997) Comparison of sinus computed tomography staging systems. Otolaryngol Head Neck Surg 117(4):372–379. https://doi.org/10.1016/S0194-5998(97)70129-3
Kohli P, Schlosser RJ, Storck K, Soler ZM (2016) Olfactory cleft computed tomography analysis and olfaction in chronic rhinosinusitis. Am J Rhinol Allergy 30(6):402–406. https://doi.org/10.2500/ajra.2016.30.4365
Soler ZM, Pallanch JF, Sansoni ER et al (2015) Volumetric computed tomography analysis of the olfactory cleft in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 5(9):846–854. https://doi.org/10.1002/alr.21552
Soler ZM, Hyer JM, Karnezis TT, Schlosser RJ (2016) The Olfactory Cleft Endoscopy Scale correlates with olfactory metrics in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 6(3):293–298. https://doi.org/10.1002/alr.21655
Schlosser RJ, Smith TL, Mace JC, Alt JA, Beswick DM, Mattos JL, Ramakrishnan V, Massey C, Soler ZM (2021) The Olfactory Cleft Endoscopy Scale: a multi-institutional validation study in chronic rhinosinusitis. Rhinology 59(2):181–190. https://doi.org/10.4193/Rhin20.307
Mattos JL, Schlosser RJ, Mace JC, Smith TL, Soler ZM (2018) Establishing the minimal clinically important difference for the Questionnaire of Olfactory Disorders. Int Forum Allergy Rhinol 8(9):1041–1046. https://doi.org/10.1002/alr.22135
Whitcroft KL, Cuevas M, Haehner A, Hummel T (2017) Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope 127(2):291–295. https://doi.org/10.1002/lary.26229
Whitcroft KL, Cuevas M, Andrews P, Hummel T (2018) Monitoring olfactory function in chronic rhinosinusitis and the effect of disease duration on outcome. Int Forum Allergy Rhinol 8(7):769–776. https://doi.org/10.1002/alr.22104
Loftus C, Schlosser RJ, Smith TL, Alt JA, Ramakrishnan VR, Mattos JL, Mappus E, Storck K, Yoo F, Soler ZM (2020) Olfactory cleft and sinus opacification differentially impact olfaction in chronic rhinosinusitis. Laryngoscope 130(10):2311–2318. https://doi.org/10.1002/lary.28332
Ahmed OG, Rowan NR (2020) Olfactory dysfunction and chronic rhinosinusitis. Immunol Allergy Clin North Am 40(2):223–232. https://doi.org/10.1016/j.iac.2019.12.013
Eibenstein A, Fioretti AB, Lena C, Rosati N, Amabile G, Fusetti M (2005) Modern psychophysical tests to assess olfactory function. Neurol Sci 26(3):147–155. https://doi.org/10.1007/s10072-005-0452-3
Olofsson JK, Gottfried JA (2015) The muted sense: neurocognitive limitations of olfactory language. Trends Cogn Sci 19(6):314–321. https://doi.org/10.1016/j.tics.2015.04.007
Philpott CM, Rimal D, Tassone P, Prinsley PR, Premachandra DJ (2008) A study of olfactory testing in patients with rhinological pathology in the ENT clinic. Rhinology 46(1):34–39
Funding
Beijing Hospitals Authority Youth Program (QML20190617), Beijing Science and Technology Nova Program (Z201100006820086), Natural Science Foundation of China (82,000,954), Beijing Hospitals Authority Clinical Medicine Development of Special Funding (XMLX202136), Beijing Hospitals Authority’ Mission Plan (SML20190601), Beijing Scholars Program (No.051) and National Key R&D Program of China (2019YFE0116000).
Author information
Authors and Affiliations
Contributions
All the authors have made substantial contributions to the conception, analysis, and interpretation of data in this article, approved the submitted version, and agreed to be personally accountable for our contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which we are not personally involved, are appropriately investigated and resolved, and the resolution documented in the literature.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, F., Wu, D. & Wei, Y. Predictive significance of the questionnaire of olfactory disorders-negative statements for olfactory loss in patients with chronic rhinosinusitis. Eur Arch Otorhinolaryngol 279, 5253–5262 (2022). https://doi.org/10.1007/s00405-022-07438-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-022-07438-z