Abstract
Purpose
To compare the clinical profile of COVID-related and non-COVID-related rhino-orbito-cerebral invasive fungal disease.
Methods
We have compared the comorbidities, clinical features, course of the disease and outcome between COVID-related and non-COVID-related acute invasive fungal rhinosinusitis (AIFRS) of the rhino-orbito-cerebral form.
Results
HbA1c and blood sugar at the time of admission were significantly higher in the non-COVID group (P < 0.05). Duration of stay, and use of steroids were significantly higher among the COVID group (P < 0.05). The period of hospital stay was significantly higher in the COVID group. The overall survival in the COVID group was 67.57%. In the non-COVID group the overall survival was 61.90%.This study found that odds of surgical treatment was significantly lower among non-survivors (P < 0.05). Similarly patients who developed stages 3 & 4 of the disease had a lower survival rate (P < 0.05).
Conclusion
Diabetes mellitus is a key risk factor for the development of AIFRS. Pre-existing, grossly uncontrolled DM was the predisposing factor in the non-COVID group. Deranged glucose profile associated with COVID illness and its treatment and immunological disturbances in a vulnerable population, contributed to the surge in cases of AIFRS in the COVID-19-related group. Patients who underwent combined medical and surgical treatment had a significantly better outcome following AIFRS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute invasive fungal rhino sinusitis (AIFRS) is a rare, fulminant infection that affects individuals in an immuno-compromised state such as malignancy, uncontrolled diabetes mellitus (DM), acquired immunodeficiency syndrome, neutropenia, chemotherapeutic drugs, iron overload, and sepsis induced immune-suppression as in COVID-19 illness [1, 2]. The causative agents are fungal organisms widely distributed in the environment that do not invade living tissues in normal circumstances. But in immune-compromised states and in the presence of predisposing factors, these opportunistic pathogens can become aggressive and produce life-threatening infections. Mucorales and Aspergillus are the most common fungal pathogens causing AIFRS. The disease is commonly acquired by inhalation of spores [1]. These pathogens especially Mucor are angio-invasive in nature leading to thrombosis of blood vessels resulting in infarction and necrosis of tissues [3]. Acute rhino-orbito-cerebral invasive fungal disease is considered the most severe and rapidly spreading form of AIFRS and is associated with variable survival rates ranging between 20 and 80% [4, 5]. A favourable prognosis is dependent on early diagnosis followed by aggressive medical and surgical management.
DM is a widely accepted risk factor for AIFRS [3, 4]. India with its high prevalence of DM is reported to be one of the countries having the heaviest burden of AIFRS cases even during the pre-COVID era [1, 6]. An alarming spurt in the number of AIFRS cases was encountered during the COVID-19 pandemic and review of existing literature shows that India contributed to 81% of the cases of COVID-19-associated rhino-orbito-cerebral mucormycosis [1]. But, did the clinical course of the disease in COVID-related AIFRS differ from that of non-COVID-related group? Till now no studies have been undertaken to compare the clinical profile of COVID-related and non-COVID-related rhino-orbito-cerebral invasive fungal disease.
In this study, we have compared the comorbidities, clinical features, course of the disease and outcome between COVID-related and non-COVID-related AIFRS.
Materials and methods
This study is a retrospective cohort analysis of AIFRS in 58 patients treated at a 595-bed tertiary care centre in Delhi, India, from Jan 2016 to June 2021. They are divided into two groups; (a) patients diagnosed with AIFRS associated with a recent or concurrent COVID-19 infection (COVID AIFRS) from December 2020 to June 2021; (b) non-COVID patients with AIFRS (non-COVID AIFRS) treated in this institution between 2016 and 2021.
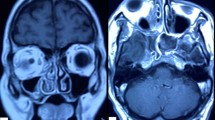
The diagnosis of COVID-19 was based on Reverse Transcription Polymerase Chain Reaction (RT-PCR) test on nasopharyngeal or oropharyngeal swabs. The cases were classified as proven or probable invasive fungal disease as per the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) guidelines [7]. Clinical features supported by diagnostic nasal endoscopy findings, radiology and positive potassium hydroxide (KOH) mount of nasal swab were identified as probable cases of AIFRS. Diagnosis of invasive fungal sinusitis confirmed by fungal culture and/or histopathological evidence of invasion of sinus mucosa was considered as a proven case. Possible cases as defined by the EORTC/MSG group were not included in this study [7]. Mucor and Aspergillus species were differentiated based on their microscopic appearance on smear/culture. Radiological investigations included Computed Tomography (CT) scans of chest and nose & paranasal sinuses (PNS). Magnetic Resonance Imaging (MRI) studies were performed when orbital or intracranial involvement was suspected.
There is no internationally accepted staging system for AIFRS based on the severity and diagnostic modalities. In this study, we have followed a staging system proposed by Honavar SG for rhino-orbito-cerebral mucormycosis [8]. This system is based on the general anatomical progression of the disease from nasal cavity to the paranasal sinuses, orbit and brain. The severity of disease in both groups can be assessed and broadly divided into four stages according to the above system.
Apart from the control of comorbid conditions, treatment included antifungal medications and surgical intervention. Amphotericin B was the preferred antifungal drug with Posaconazole and/or Voriconazole in some cases. Surgical debridement included endoscopic, open or combined approaches with multiple sittings when required. Management of intracranial and ophthalmic complications was carried out in consultation with neurology and ophthalmology colleagues.
Patient data were retrieved from inpatient records, discharge summaries and computerised hospital information system. Demographics, clinical presentation, risk factors, comorbidities, radiological findings, microbiology and pathology investigation data, staging of the disease, medical and surgical treatment, duration of stay in the hospital and final outcome were analysed and compared.
Statistical analysis
All statistical analyses were performed using MedCalc software Ltd and the odds ratio calculator. The data were summarised with the mean and standard deviation for continuous variables and as numbers with percentages for qualitative variables. The clinical data and other variables were compared using an unpaired student’s t test for continuous variables and a chi-squared test for qualitative variables. A univariate logistic analysis was fitted for each variable. Variables with a P value < 0.05 were considered statistically significant.
Results
In this retrospective analysis, the COVID AIFRS group comprised 37 patients and the non-COVID AIFRS group of 21 patients. Demographic data, associated comorbidities, stage of disease, treatment, hospital stay and outcomes of both groups of AIFRS are listed in Table 1. In the COVID group, 25 patients had DM of which 3 were of recent on set. Systemic corticosteroids (either oral or intravenous or both) were used in 29 (78.38%) patients in COVID group of patients, while 8 (21.62%) did not receive corticosteroids in any form. In the COVID group, (29.73%) (11 patients) did not have DM, although they had received steroids. One patient had no apparent risk factor other than COVID illness. All patients in the non-COVID group were diabetic and three patients developed DM following corticosteroid therapy prior to the onset of AIFRS. HbA1c and blood sugar at the time of admission were significantly higher in the non-COVID group and duration of stay, and use of steroids were significantly higher among the COVID group (P < 0.05). Other features were comparable in both groups of patients.
In the COVID group, we have included only patients with a positive COVID-RT-PCR test. Among them, 37.84% (14) were having an active infection and the remaining patients were in the post-COVID state. According to the EORTC/MSG guidelines, 16.22% (6) patients of the COVID group had a probable diagnosis and 83.78% (31) patients had proven diagnosis of AIFRS. The causative fungus belonged to Mucor species in 15 cases while Aspergillus was detected in 15 cases and 7 cases had a mixed fungal infection in the COVID-group. In the non-COVID group, 19.04% (4) were probable cases while 80.95% (17) were proven cases of AIFRS. In this group, 17 cases were of Mucor species (80.96%), 2 (9.52%) were of Aspergillus and two (9.52%) were of dual origin. In the present study, Mucor species was identified in 58.62%, Aspergillus fumigatus in 32.76% and mixed infection in 29.31% of cases.
Based on clinico-radiological and endoscopic features clinical staging was done (Table 2). Bilateral involvement of nose and paranasal sinuses was present in 8 cases in the COVID group and six cases in the non-COVID group. Maxillary sinus was the most frequently involved site followed by ethmoid and sphenoid sinuses in both groups. Extension of disease to the orbit was present in five cases in COVID-group and six cases in non-COVID group. Altered sensorium was the most common symptom in cases with intracranial extension among both groups, followed by evidence of meningitis and cavernous sinus thrombosis.
Along with the stabilization of haemodynamic parameters and control of any associated comorbidities, treatment for AIFRS included antifungal treatment and surgical debridement. In 78.38% (29) patients of the COVID group, initial treatment was with liposomal Amphotericin B during the active phase of AIFRS followed by oral Posaconazole for 6–16 weeks during the recovery phase of illness. Oral Voriconazole was administered for six patients. Oral Posaconazole was administered in two patients with kidney failure. Endoscopic surgical debridement was done in 72.97% (27) patients of the COVID group. Combined endoscopic and open approaches were utilized in three patients of this group. In the non-COVID group, all patients received liposomal Amphotericin B and 76.19% (16) patients underwent endoscopic surgical debridement as well. Surgical debridement was based on the site and extent of involvement. It included a variety of procedures like middle and inferior turbinectomy, partial septectomy, middle meatal antrostomy, ethmoidectomy, sphenoidotomy, Draf I procedure, endoscopic evacuation of subperiosteal orbital abscess, orbitotomy, orbital decompression, endoscopic and open medial maxillectomy. Skin necrosis due to invasive fungal infection was managed by debridement followed by secondary suturing.
The overall survival in the COVID group was 67.57%. In the non-COVID-group the overall survival was 61.90%. Out of the 12 people who expired 7 died due to other COVID-related respiratory and cardiac complications after clinical improvement from AIFRS. In the non-COVID group, all patients who expired were still in the active phase of AIFRS.
Table 3 shows univariate odds ratios (± 95% confidence interval) of patients between survivors and non-survivors. This study found that odds of surgical treatment was significantly lower among non-survivors (P < 0.05). Similarly patients who developed stages 3 & 4 of the disease had a lower survival rate (P < 0.05).
Discussion
AIFRS is an opportunistic infection with a high mortality rate that affect the nose, paranasal sinuses, orbit, CNS, lung and skin. The current study revealed a sudden surge in the number of cases during the second wave of COVID-19. In the pre-COVID period the total number of AIFRS cases treated in this institution were 4–6 per year. But in contrast, the number of COVID- associated AIFRS in the 6 months period from December 2020 to June 2021 rose up to 37. Demographic profile of the patients in our study was consistent with previous studies [1, 4] with a male predilection in both groups.
Risk factors of AIFRS were also similar in both groups of patients. Diabetes mellitus (DM) has been recognised as an independent risk factor for AIFRS especially when poorly controlled or when associated with diabetic ketoacidosis [3,4,5, 9, 10]. Apart from rising glucose levels, acidosis raises free iron levels, further favouring invasive fungal growth [1]. India is already having the second largest population with DM in the world with 77million diabetics [11]. Hence the spurt in AIFRS in an already vulnerable population during COVID-19 illness with its associated immunological derangement is not surprising.
Uncontrolled DM remained the most common co-morbidity in both the groups of the current study. Some of the recent studies have implicated COVID-19 infection in the genesis or worsening of hyperglycemia [12, 13]. In the non-COVID group, all patients were long-term diabetics, except one case of freshly detected DM. Between the COVID and non-COVID groups, we observed a significant difference between HbA1C values at the time of admission. COVID group HbA1C—8.62 ± 3.18 and non-COVID group HbA1C—11.35 ± 3.17. Markedly elevated HbA1c values (P < 0.05) implicates a pre-existing, grossly uncontrolled DM as the predisposing factor for AIFRS in the non-COVID group. Blood sugar at the time of admission was also significantly high in the non-COVID group (P < 0.05). In the COVID group, marginally elevated HbA1C suggests a relatively better glycemic control prior to the illness. The use of steroids was also found to be significantly higher among the COVID group (P < 0.05). Addition of steroids as part of the treatment in critically ill COVID patients might have led to reduced phagocytic activity of WBC, uncontrolled hyperglycemia and precipitation of diabetic ketoacidosis [14]. Hence COVID illness and its treatment must have led to a recent hyperglycemic state favouring invasive fungal infection.
COVID illness in itself has been identified as a predisposing condition for AIFRS. Triggering factors like epithelial damage of the respiratory tract and immune dysfunctions also might have contributed to the increase in number of AIFRS cases [15, 16]. Further, it has been postulated that COVID-19 infection can induce lymphopenia and may affect CD4+ and CD8+ T-cell counts [2, 17, 18]. Here, both groups of patients have exhibited lymphopenia: 23 (62.16%) in the COVID–group and 14 (66.67%) in the non-COVID group, facilitating secondary fungal infections. We observed a ~ 15-fold increase in AIFRS cases from December 2020 to June 2021 in our study. This alarming rise in AIFRS cases has coincided with the steep rise of COVID-19 illness in Delhi. The plausible explanation for this sudden surge in AIFRS cases could be COVID-19 precipitated immune dysfunctions.
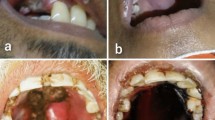
Clinical presentation of disease was similar in both groups of AIFRS in this study. Previous studies on non-COVID AIFRS also have shown concomitant features [4, 5, 9, 19]. Diagnostic nasal endoscopy was helpful in the evaluation and staging of the disease and better sampling of material for microbiological examination. The presence of necrotic mucosa and mucopus in the middle meatus was suggestive of AIFRS [20]. The most common presenting symptoms were headache, facial pain, periorbital swelling and proptosis. Similar to previous studies [3, 5, 9, 20], extension of disease to the cranial cavity and orbit (Stages 3 & 4) was associated with a poorer prognosis in this study also (Table 3).
Radiological imaging is often complementary to clinical evaluation. CT and MRI proved to be helpful in assessing the extent of disease and in the identification of complications and is indispensable for surgical planning. Although CT paranasal sinuses shows only non-specific inflammatory changes in the early stages, it is an effective screening tool in patients at risk of acute invasive fungal sinusitis [21]. MRI was useful in detection of complications like orbital cellulitis, cavernous sinus thrombosis and internal carotid artery thrombosis. Microbiological investigation included microscopic examination of nasal smear on KOH and fungal culture. Mucor, Rhizopus and Aspergillus are the fungal species reported to cause AIFRS [22, 23]. The causative fungal organism were identified based on the characteristics of the hyphae including diameter, presence or absence of septa, branching angle and pigmentation. The spectrum of fungal infection in the COVID group; Aspergillus alone was identified in 15 patients (40.54%), Mucor alone in 15 cases (40.54%) and mixed infection in 7 cases (18.92%). But the pattern was different in the non-COVID group: Mucor 17 cases (80.56%), Aspergillus in 2 cases (9.52%) and mixed infection in 2 cases (9.52%). A similar, preponderance of Aspergillus infection was observed in COVID-19-associated invasive pulmonary fungal infection in several studies [24, 25]. Histopathological examination of tissue from the nose and sinuses was done for further confirmation. All patients in this study had a proven/probable diagnosis of AIFRS.
We experienced several challenges in the management of AIFRS patients in our study, especially in the COVID-associated group. This includes a delay in seeking health care, poor general condition of the patient, difficulty in establishing the diagnosis, poor availability and cost of antifungal drugs in the wake of an unexpected surge of cases during the COVID wave and delay in surgical management. Treatment included antifungal medications and surgical intervention together with control of comorbid conditions like diabetes mellitus. Liposomal Amphotericin B was the preferred drug and Posaconazole was used in cases with deranged kidney function. Voriconazole was used in Aspergillus infections. The first dose of antifungal medications were started empirically in all patients with strong clinical and diagnostic nasal endoscopic features suggestive of AIFRS. KOH mount results were obtained within 4 h of sampling which enabled us to start these medications in all clinically negative but smear-positive fungal cases also.
In the current study, surgical debridement was found to be a statistically significant factor in the survival of patients with AIFRS (P < 0.05). Surgery was carried out within 24 h of clinical suspicion and included endoscopic, open, and combined approaches with multiple sittings when required. Early surgery is a vital adjunct to medical management since antifungal medications cannot enter devascularised tissues and surgery aids in reducing the load of fungal and necrotic tissues. Surgery also helps in the re-establishment of ventilation of the sinuses and provides material for histopathological confirmation of the disease. Although timely surgical debridement is of crucial importance in the prognosis, the benefits of aggressive surgical debridement need to be weighed against the hazards of general anaesthesia and overall prognosis in critically ill patients. Additionally, endoscopic sinus surgery carries a high risk of significant aerosol production [26, 27] during the procedure. Since 37.84% of the patients who underwent endoscopic procedures still had active COVID-19 infection, the risk of transmission to health-care workers in the operating and recovery rooms was very high. We have tried to minimise this risk using personal protective equipment, a dedicated operation theatre and surgical instruments, restricting the number of health-care workers in the operation theatre and avoiding the use of powered instruments whenever possible.
The period of hospital stay was significantly higher in the COVID group, although it could be attributed to other COVID-related morbidities also. In previous studies, the overall mortality of patients with AIFRS remained high, with only half of the patients surviving the illness [4, 19]. Our study does not show any significant difference in the clinical course of AIFRS in COVID and non-COVID patients. The relatively better survival rate in this study could be due to early diagnosis, early use of antifungal medications good diabetic control and aggressive surgical debridement.
Strengths and limitations
The main strength of this study is that all patients had microbiological/histopathological evidence of AIFRS. The evaluation of HbA1C and blood sugar at the time of admission enabled us to assess the evolution DM in both groups of patients. The limitations of this study are the relatively limited patient number, single tertiary care center experience, and short-term follow-up. Hence, a multivariate logistic regression analysis could not be carried out in this study. Outcomes were difficult to assess in the COVID group since many patients continued to have other COVID-related morbidities even after recovery from AIFRS.
Conclusions
Clinical presentation was similar in both COVID and non-COVID groups of patients and DM remained a key risk factor in this study. Pre-existing, grossly uncontrolled DM was the predisposing factor in the non-COVID group of patients. Deranged glucose profile associated with COVID illness and its treatment and immunological disturbances in a vulnerable population, contributed to the surge in cases of AIFRS during the COVID-19 pandemic in India. Compared to the non-COVID group, there was higher proportion of Aspergillus infection in COVID-associated invasive fungal rhinosinusitis. Patients who underwent combined medical and surgical management had a significantly better outcome following AIFRS.
References
Singh AK, Singh R, Joshi SR, Misra A (2021) Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in india. Diabetes Metab Syndr. https://doi.org/10.1016/j.dsx.2021.05.019
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immune-suppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13:862–874. https://doi.org/10.1038/nri3552
Prakash H, Chakrabarti A (2019) Global epidemiology of mucormycosis. J Fungi 5:26. https://doi.org/10.3390/jof5010026
Turner JH, Soudry E, Nayak JV, Hwang PH (2013) Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope 123:1112–1118
Monroe MM et al (2013) Invasive fungal rhinosinusitis: a 15-year experience with 29 patients. Laryngoscope 123:1583–1587
Mucormycosis—WHO World Health Organization. https://www.who.int>coronavirus-disease-(COVID-19). Accessed 1 Feb 2022
De Pauw B, Walsh TJ, Donnelly JP et al (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clin Infect Dis 46:1813–1821
Honavar SG (2021) Code Mucor: guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol 69:1361–1365. https://doi.org/10.4103/ijo.IJO_1165_21
Kursun E, Turunc T, Demiroglu YZ, Alışkan HE, Arslan AH (2015) Evaluation of 28 cases of mucormycosis. Mycoses 58:82–87
Bellazreg F, Hattab Z, Meksi S et al (2015) Outcome of mucormycosis after treatment: report of five cases. New Microbes New Infect 6:49–52
https://idf.org/our-network/regions-members/south-east-asia/members/94-india.html. Accessed 2 Jan 2022
Metwally AA, Mehta P, Johnson BS, Nagarjuna A, Snyder MP (2021) COVID-19—induced new-onset diabetes: trends and technologies. Diabetes 70:2733–2744. https://doi.org/10.2337/dbi21-0029
Al-kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N (2021) COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2021.644095
Rudramurthy SM, Hoenigl M, Meis JF et al (2021) ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses. https://doi.org/10.1111/myc.13335
Ramaswami A, Sahu AK, Kumar A, Suresh S (2021) COVID-19-associated mucormycosis presenting to the Emergency Department—an observational study of 70 patients. QJM Int J Med. https://doi.org/10.1093/qjmed/hcab190
Sebastian SK, Kumar VB, Gupta M, Sharma Y (2021) COVID associated invasive fungal sinusitis. Indian J Otolaryngol Head Neck Surg 25:1–4. https://doi.org/10.1007/s12070-021-02471-6Accessedon29/01/2022
Shweta MR, Supriya PS, Laxminarayana S, Vineeth VK (2021) COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world. BMJ Case Rep 14(4):e241663. https://doi.org/10.1136/bcr-2021-241663
Illg Z, Muller G, Mueller M, Nippert J (2021) Analysis of absolute lymphocyte count in patients with COVID-19. Am J Emerg Med 46:16–19. https://doi.org/10.1016/j.ajem.2021.02.054
Bakhshaee M, Bojdi A, Allahyari A et al (2016) Acute invasive fungal rhinosinusitis: our experience with 18 cases. Eur Arch Otorhinolaryngol 273:4281–4287
Meher R, Wadhwa V, Kumar V, Phanbuh DS et al (2022) COVID associated mucormycosis: a preliminary study from a dedicated COVID hospital in Delhi. Am J Otolaryngol 43(1):103220. https://doi.org/10.1016/j.amjoto.2021.103220
Sen M, Honaver SG, Bansal R (2021) Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India-Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), report 1. Indian J Ophthalmol 69(7):1670–1692
Middlebrooks EH, Frost CJ, Jesus ROD, Massini TC, Schmalfuss IM, Mancuso AA (2015) Acute invasive fungal rhinosinusitis: a comprehensive update of CT findings and design of an effective diagnostic imaging model. Am J Neuroradiol 36:1529–1535
Wandell GM et al (2018) A multi-institutional review of outcomes in biopsy-proven acute invasive fungal sinusitis. Int Forum Allergy Rhinol 8:1459–1468
Lai CC, Yu WL (2021) COVID-19 associated with pulmonary aspergillosis: a literature review. J Microbiol Immunol Infect 54(1):46–53. https://doi.org/10.1016/j.jmii.2020.09.004
Montrucchio G, Lupia T, Lombardo D et al (2021) Risk factors for invasive aspergillosis in ICU patients with COVID-19: current insights and new key elements. Ann Intensive Care 11:136
El-Kholy NA, Abd El-Fattah AM, Khafagy YW (2021) Invasive fungal sinusitis in post COVID-19 patients a new clinical entity. Laryngoscope. https://doi.org/10.1002/lary.2963
Givi B, Schiff BA, Chinn SB et al (2020) Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg 146:579–584
Funding
We have not received any funding or financial support from any source for the conduct of this study. The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Contributions
SKS: Substantial contributions to the conception of the work, the acquisition, analysis, interpretation, critical analysis of data and drafting. Approved the version to be published and agreed to be accountable for all aspects of the work. SP: Substantial contributions to the acquisition, analysis, interpretation and drafting of data approved the version to be published and agreed to be accountable for all aspects of the work. YS: Substantial contributions to the interpretation, analysis and revision of data. Approved the version to be published and agreed to be accountable for all aspects of the work. RKJ: Substantial contributions to the acquisition and analysis, of data. Approved the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
Institutional ethics committee clearance obtained. No: SSHEC/R0192. This study is a retrospective analysis. Hence it does not have a CTRI Number. All procedures performed in this study are in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its amendments. No information revealing the participants identity is included in the manuscript. Informed consent has been waived by the ethics committee in view of the retrospective nature of the study and all the procedures being performed were part of the routine care. All authors declare that all data and materials as well as software application comply with field standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sebastian, S.K., Ponnuvelu, S., Sharma, Y. et al. A comparative study on the clinical profile of COVID-related and non-COVID-related acute invasive fungal rhino sinusitis. Eur Arch Otorhinolaryngol 279, 5239–5246 (2022). https://doi.org/10.1007/s00405-022-07402-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-022-07402-x