Abstract
Purpose
We performed a meta-analysis to compare the efficacy and safety of induction chemotherapy (IC) followed by cisplatin-based concurrent chemoradiotherapy (CCRT) versus cisplatin-based CCRT for patients with locally advanced nasopharyngeal carcinoma (LA-NPC).
Methods
We systematically searched PubMed, the Cochrane Library and Embase until February 29, 2020, for eligible randomized controlled trials (RCTs). The quality of the studies was assessed with the Cochrane Collaboration risk of bias tool. The following outcomes of interest were analyzed: 1) progression-free survival (PFS); 2) overall survival (OS); 3) distant metastasis-free survival (DMFS); 4) locoregional failure-free survival (LRFFS); and 5) any grade 3 or 4 treatment-related adverse events (AEs). The data were pooled with the use of hazard ratios (HRs) or odds ratios (ORs). Subgroup, heterogeneity and sensitivity analyses were performed.
Results
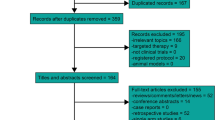
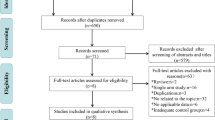
After screening all studies selected from the initial search, seven trials with 2233 patients met the inclusion criteria. Compared to cisplatin-based CCRT alone, there was reliable evidence that IC significantly improved PFS (HR 0.65, 95% CI 0.55–0.75, P < 0.00001), OS (HR 0.61, 95% CI 0.45–0.83, P = 0.002), DMFS (HR 0.65, 95% CI 0.53–0.79, P < 0.0001) and LRFFS (HR 0.68, 95% CI 0.53–0.88, P = 0.003) and was associated with a markedly increased risk of AEs (grade 3/4) during the IC and CCRT phases. No significant difference in the incidence of late AEs (grade 3/4) was observed between different arms.
Conclusion
Compared to cisplatin-based CCRT alone, IC followed by cisplatin-based CCRT confers real and substantial favorable survival outcomes with controllable toxicities. Therefore, it is reasonable to define IC followed by cisplatin-based CCRT as one of the standard treatment strategies for patients with LA-NPC.



Similar content being viewed by others
References
Chen YP, Chan ATC, Le QT et al (2019) Nasopharyngeal carcinoma. Lancet (London, England) 394(10192):64–80
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Ferlay J, Colombet M, Soerjomataram I et al (2018) Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103:356–387
Mao YP, Xie FY, Liu LZ et al (2009) Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys 73(5):1326–1334
Wu F, Wang R, Lu H et al (2014) Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol 112(1):106–111
Colevas AD, Yom SS, Pfister DG et al (2018) NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw 16(5):479–490
Hui EP, Ma BB, Leung SF et al (2009) Randomized Phase II Trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 27(2):242–249
Fountzilas G, Ciuleanu E, Bobos M et al (2012) Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann oncol 23(2):427–435
Lee AWM, Ngan RKC, Tung SY et al (2015) Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 121(8):1328–1338
Huang PY, Zeng Q, Cao KJ et al (2015) Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: a single-institution experience from an endemic area. Eur J Cancer 51(13):1760–1770
Tan T, Lim WT, Fong KW et al (2015) Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 91(5):952–960
Frikha M, Auperin A, Tao Y et al (2018) A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006–02). Ann oncol 29(3):731–736
Hong RL, Hsiao CF, Ting LL et al (2018) Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann oncol 29(9):1972–1979
Zhang Y, Chen L, Hu GQ et al (2019) Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 381(12):1124–1135
Li WF, Chen NY, Zhang N et al (2019) Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J cancer 145(1):295–305
Yang Q, Cao SM, Guo L et al (2019) Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer 119:87–96
Adelstein DJ (2012) Clinical trial design in head and neck cancer: what has the oncologist learned? Lancet Oncol 13(7):e318–323
Higgins JPT, López-López JA, Becker BJ et al (2019) Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health 4:e000858
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100
Higgins JPT, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Begg CB, Mazumdar M (1995) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Cao SM, Yang Q, Guo L et al (2017) Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer 75:14–23
Ma J, Chen NY, Zhang N et al (2014) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: preliminary results of a phase 3 multicentre randomised controlled trial. Eur J Cancer 50:e3–e4
Sun Y, Li WF, Chen NY et al (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17(11):1509–1520
Yan M, Al K, Siu LL et al (2015) Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis. Eur J Cancer 51(12):1570–1579
Blanchard P, Lee A, Marguet S et al (2015) Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 16(6):645–655
Wang M, Tian H, Li G et al (2016) Significant benefits of adding neoadjuvant chemotherapy before concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Oncotarget 7(30):48375–48390
Liang ZG, Zhu XD, Tan AH et al (2013) Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy with or without adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: meta-analysis of 1,096 patients from 11 randomized controlled trials. Asian Pac J Cancer Prev 14(1):515–521
Tan TH, Soon YY, Cheo T et al (2018) Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: a systematic review and meta-analysis. Radiother Oncol 129(1):10–17
Wang P, Zhang M, Ke C et al (2020) The efficacy and toxicity of induction chemotherapy plus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Medicine 99(10):e19360
Zhang B, Mo Z, Du W et al (2015) Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis. Oral Oncol 51(11):1041–1046
Vermorken JB, Remenar E, Van HC et al (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357(17):1695–1704
Posner MR, Hershock DM, Blajman CR et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357(17):1705–1715
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MT, ZJ and JZ. The first draft of the manuscript was written by Min Tang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, M., Jia, Z. & Zhang, J. The evaluation of adding induction chemotherapy to concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma: a meta-analysis. Eur Arch Otorhinolaryngol 278, 1545–1558 (2021). https://doi.org/10.1007/s00405-020-06218-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-06218-x