Abstract
Objective
Nasopharyngeal carcinoma (NPC) is a common malignancy in Southern China and Southeast Asia. Genetic susceptibility is a major contributing factor in determining the individual risk of NPC in these areas. To test the association between NPC and variants in regenerating gene 1A (REG1A), we conducted a hospital-based case–control study in a Cantonese-speaking population from Guangdong province.
Methods
We endeavored to determine whether genetic variants of the REG1A gene were associated with the risk of NPC amidst the Cantonese population in a hospital-based case–control study using polymerase chain reaction-restriction and direct sequencing analysis in 211 NPC patients and 150 healthy controls. The association between NPC risk and the 14C/T, 20C/T, 369G/T, 1201A/G, and 2922C/T polymorphisms was examined after adjustment for age and sex.
Results
We found an increased risk of developing NPC in individuals with REG1A 2922C/T variant genotype (p = 0.003, OR 0.419, 95% CI 0.235–0.746), and after adjustment for sex and age (p = 0.003, OR 0.406, 95% CI 0.226–0.732). No association between other polymorphisms (14C/T, 20C/T, 369G/T, and 1201A/G) and the risk of NPC was observed, before or after adjustment for age and sex.
Conclusion
Our findings suggest that the REG1A 2922C/T polymorphism is associated with an increased risk of developing NPC in a Cantonese population from Guangdong province. Larger studies are required to confirm our findings and unravel the underlying mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) originates from the epithelial lining of the nasopharynx [1]. It is characterized by a higher incidence in Southern China and Southeast Asia, at 20–30 per 100,000 people [2]. The etiology of NPC is not clear, but genetic susceptibility, Epstein–Barr virus (EBV) infection and environmental factors are predisposing factors for NPC [3, 4]. Different individuals exposed to the same environmental factors will have distinct presentation, suggesting that genetic variation is the key risk factor for the development of NPC [5].
Many genetic studies have been conducted to address susceptibility genes for NPC and, using linkage approach or association analyses, some genes have been identified. −842G>C and −667C>T in PIN1 promoter variants [6], Filaggrin gene (FLG) single-nucleotide polymorphism (SNP) loci (rs3126085, K4671X) [7], Cyclin D1 (CCND1) [8], and the DNA repair genes hOGG1 and XRCC1 [9] seem to contribute to NPC. Some studies have indicated that SNPs are associated with treatment outcomes and prognosis in NPC. SNPs such as rs2074549 in valosin-containing protein, rs7566 in CANX, rs2528521 in CALCR and rs9344 in CCND1 may serve as predictors for clinical outcomes of chemoradiotherapy in Chinese NPC patients [10, 11]. SNPs like rs1800541, rs2071942 and rs5370 in EDN1, rs5333 in EDNRA and VEGF −460T/C may serve as useful biomarkers for predicting the outcomes of NPC patients [12, 13]. Another recent study has shown that genetic polymorphisms in MCP-1 and HLA-G are associated with NPC prognosis [14, 15]. Despite these advances, the alleles that account for most of the genetic susceptibility to NPC remain undiscovered.
The regenerating gene (REG) was originally isolated from a complementary DNA (cDNA) library derived from regenerating rat’s pancreatic islets and consists of a group of acute phase reactants, lectins, anti-apoptotic factors, or growth factors for pancreatic β-islet cells and epithelial cells in the digestive system. Until now, 17 members of the REG family have been identified and classified into four classes (Reg I–IV) [16]. REG1A is one of the members of the human REG1 family, and has six exons encoding a 166-amino-acid protein and a 22-amino-acid signal sequence [17]. In humans, REG1A and the other members of the REG1 family (REG1B, RS (REG-related sequence), and PAP genes) are clustered in a 95-kb region on chromosome 2p12.
REG1A is involved not only in inflammatory diseases, but also in gastroenterological carcinogenesis, including the stomach [18], colon [19], and pancreas [20]. Meanwhile, evidence has revealed that REG1A is also related to breast cancer [21], lung cancer [22], recurrence of bladder cancer [23], hepatocellular carcinoma metastasis [24], and prognosis of head and neck tumors [25]. Some recent studies also demonstrated that REG1A expression was associated with chemoradiotherapy outcome in patients with esophageal squamous cell cancer [26]. A previous study by our group showed that REG1A overexpression was associated with NPC progression and prognosis [27]. Studies had seldom been conducted concerning the REG1A polymorphisms in relation to cancer.
This has provided a molecular basis for determining possible associations of REG1A polymorphisms and NPC predisposition. In the present case–control study, we first sequenced all six exons of the REG1A gene and evaluated for the potential link between the REG1A polymorphisms and the risk of NPC among Guangdong Cantonese. We found that the REG1A 2922C/T variants are associated with an increased risk of NPC and may be a novel biomarker for the screening and early diagnosis of NPC.
Materials and methods
Subjects
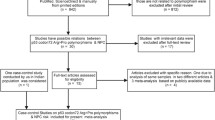
A total of 211 patients with NPC were enrolled in the study at the Chinese Sciences Academy University, Shenzhen Hospital in Guangdong Province from January 2012 to December 2016. All patients were diagnosed based on pathology.
In addition, 150 healthy subjects were selected randomly for the control group from the health screening program participants during the same period as the patients were enrolled. Individuals with a history of cancer or those related to the included patients were excluded. The controls and patients were frequency-matched for sex, age, and residential area.
The Institutional Ethics Committee of Chinese Science Academy University, Shenzhen Hospital approved this study. Informed consent was obtained from all the subjects included in this study before any study procedure.
DNA extraction and genotyping
Genomic DNA was extracted from the NPC tissues and peripheral venous blood samples at the time of enrollment for genotyping using the QIAamp DNA FFPE Tissue Kit (Qiagen, Germany), according to the manufacturer’s instructions, at Ubiolab Genetics Technology Ltd. (Beijing, China).
Based on the DNA sequences (GenBank ID: 5967) of the human REG1A gene, six pairs of specific polymerase chain reaction (PCR) primers, covering the six exons, were designed using the Primer Premier v5.0 software to amplify the exons (Table 1).
Amplification and sequencing
The first PCR reaction was carried out with 2.5 μL of 10× buffer Gold (Applied Biosystems, USA), 200 μM each dNTP, 1.5 μM each primer, 1.25 U of AmpliTaq Gold Polymerase (Applied Biosystems, USA) and about 20 ng of DNA, in 25 μL total volume. Thermal cycling was performed in a Veriti system (Applied Biosystems, USA), and consisted of an initial 5 min denaturation step at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at the 58 °C and 40 s extension step at 72 °C, followed by 5 min at 72 °C.
The second PCR was carried out with 2.5 μL of 10× buffer Gold (Applied Biosystems, USA), 200 μM each dNTP, 1.5 μM each primer, 1.25 U of AmpliTaq Gold Polymerase (Applied Biosystems, USA) and about 2 μL product of the first amplification, in 25 μL total volume. Thermal cycling was performed in a Veriti system (Applied Biosystems, USA), and consisted of an initial 5 min denaturation step at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at the 58 °C and 40 s extension step at 72 °C, followed by 5 min at 72 °C.
The final PCR products were cleaned up using the QIAquick PCR purification kit (Qiagen, Germany) and then sequenced using the BigDye Terminator kit v3.1 (Applied Biosystems, USA) according to the manufacturer’s protocol. The REG1A SNPs Reg1A-14 (14C/T; exon 1), rs10165462 (20C/T; exon 1), rs117580393 (369G/T; exon 2), rs768985544 (1201A/G; exon 3), and rs12072 (2922T/C; exon 6) were examined.
Statistical analysis
Genotype and minor allele frequency for the REG1A were counted, and the Hardy–Weinberg equilibrium was tested using the Chi-square test and the gPlink and Haploview software. The associations of REG1A polymorphisms with the risk of NPC were examined with univariable and multivariable logistic regression analyses (unconditional, or after adjustment for age and sex), and presented as odds ratio (OR) and 95% confidence interval (CI), using the SPSS software (version 19.0, IBM, Armonk, NY, USA). The minor allele frequency was calculated. Two-sided p values < 0.05 were considered statistically significant.
Results
Characteristics of NPC patients and controls
There were 172 male and 39 female patients, and the average age was 49.2 ± 5.8 years. There were 110 male and 40 female controls, and the average age was 48.3 ± 5.2. There were no significant differences between patients and controls with regard to age and sex (both p > 0.05).
DNA sequencing revealed the genotype variations in the exons 1, 2, 3 and 6 of REG1A gene (Table 2). The distribution of each REG1A gene polymorphism in total samples conformed to the Hardy–Weinberg equilibrium (p = 1 for REG1A-14, p = 1 for rs117580393, p = 1 for rs768985544 and p = 0.06995 for rs12072; supplementary table 1), except for re10165462 (p = 0.002; supplementary Table 1). In the genotype comparison using Chi-square test, only rs12072 showed a statistical difference between the cases and the controls (p = 0.0005) (Table 2), which was consistent with its different distributions in the case group (equilibrium) (p = 0.06995; supplementary table 1) versus the control group (p = 0.00066; supplementary Table 1).
Association of REG1A polymorphisms with risk of NPC
Table 3 presents the multivariable logistic regression analyses between the genotypes and the risk of NPC. The REG1A 14C/T, 20C/T, 20T/T, 369G/T, 1201A/G, and 2292C/C genotypes were not associated with the risk of NPC, either before or after adjustment for age and sex. On the other hand, the REG1A 2922C/T polymorphism was significantly associated with the risk of NPC [p = 0.003 (OR 0.406, 95% CI = 0.226–0.732)]. This association was still statistically significant after adjusting for age and sex [p = 0.003 (OR 0.419, 95% CI 0.235–0.746)] (Table 3).
Discussion
Genetic variations result in differences in gene function, which in turn lead to different susceptibility to diseases. Many studies have been conducted to investigate the genetic variants that contribute to NPC susceptibility, but the molecular mechanisms are not completely understood.
Genetic variations of the exons in the REG1A gene have been examined in small numbers of studies using a combination of restriction fragment length polymorphism (RFLP), single-strand conformation polymorphism (SSCP) and sequencing techniques, and no mutations were identified in the coding regions of REG1Α gene and to be associated with the development and progression of several types of pancreatic diseases [28, 29]. Those studies were restricted to a possible role of regulatory variants and some coding regions in the REG1A gene. Another study analyzed the coding region in 12 Thai patients with fibrocalculous pancreatic diabetes and 22 controls, but T-385C, a polymorphism in exon 1 (5′UTR) has been missed due to the inherent limitations of techniques like SSCP, and because not all coding region can be detected in some cases [29]
In the present study, to the best of our knowledge, we are the first to examine the REG1A variants in cancer by an extensive analysis of the six exons of REG1A using direct sequencing. Our observations revealed a statistically significant difference in allele frequency between these groups for 2922C/T, suggesting that an association exists between the allelic variations in REG1A and NPC susceptibility. Individuals with the 2922C/T genotype will have a higher risk for developing NPC than the ones who do not have it (OR 0.419, 95% CI 0.235–0.746).
Evidence suggests that genetic variants may enhance the promoter activity and can affect their biogenesis, processing, and target site binding in a variety of ways [30]. The mechanism of the 2922C/T in the development of NPC needs subsequent further exploration.
Although our study has shown that one polymorphism in the REG1A gene is associated with the development of NPC in a Cantonese-speaking population, some limitations should be considered. The sample size of the study was relatively small (211 patients and 150 controls), and perhaps resulted in rs10165462 not respecting the Hardy–Weinberg equilibrium. In addition, other factors associated with NPC (such as smoking, alcohol and Epstein–Barr virus infection) and the interactions with each other were not accounted for. Those factors would bias the observation. Therefore, large patient cohort studies are required to confirm our results.
In summary
Our preliminary study provides the first evidence that the heterozygote 2922C/T polymorphism in the REG1A gene may be moderately associated with an increased risk for NPC. The identified REG1A rs12720T/C may be a predictive marker to identify patients with a high risk for NPC in a Southern Chinese population. The exact mechanism that polymorphism of REG1A enhances the risk of NPC requires further investigation.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Yoshizaki T, Ito M, Murono S, Wakisaka N, Kondo S, Endo K (2012) Current understanding and management of nasopharyngeal carcinoma. Auris Nasus Larynx 39(2):137–144
Liu Z, Fang F, Chang ET, Ye W (2015) Cancer risk in the relatives of patients with nasopharyngeal carcinoma—a register-based cohort study in Sweden. Br J Cancer 112(11):1827–1831
Chen CJ, Liang KY, Chang YS, Wang YF, Hsieh T, Hsu MM, Chen JY, Liu MY (1990) Multiple risk factors of nasopharyngeal carcinoma: Epstein–Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res 10(2B):547–553
Schottenfeld D, Fraumeni JF (2006) Cancer epidemiology and prevention. Oxford University Press, Oxford
Zeng L, Luo S, Li X, Lu M, Li H, Li T, Wang G, Lyu X, Jia W, Dong Z, Jiang Q, Shen Z, Huang GL, He Z (2017) Functional PIN1 promoter polymorphisms associated with risk of nasopharyngeal carcinoma in Southern Chinese populations. Sci Rep 7(1):4593
Yang Y, Liu W, Zhao Z, Zhang Y, Xiao H, Luo B (2017) Filaggrin gene polymorphism associated with Epstein–Barr virus-associated tumors in China. Virus Genes 53(4):532–537
Deng L, Zhao XR, Pan KF, Wang Y, Deng XY, Lü YY, Cao Y (2002) Cyclin D1 polymorphism and the susceptibility to NPC using DHPLC. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 34(1):16–20
Cho EY, Hildesheim A, Chen CJ, Hsu MM, Chen IH, Mittl BF, Levine PH, Liu MY, Chen JY, Brinton LA, Cheng YJ, Yang CS (2003) Nasopharyngeal carcinoma and genetic polymorphisms of DNA repair enzymes XRCC1and hOGG1. Cancer Epidemiol Biomark Prev 12(10):1100–1104
Guo XB, Ma WL, Liu LJ, Huang YL, Wang J, Huang LH, Peng XD, Yin JY, Li JG, Chen SJ, Yang GP, Wang H, Guo CX (2017) Effects of gene polymorphisms in the endoplasmic reticulum stress pathway on clinical outcomes of chemoradiotherapy in Chinese patients with nasopharyngeal carcinoma. Acta Pharmacol Sin 38(4):571–580
Guo C, Huang Y, Yu J, Liu L, Gong X, Huang M, Jiang C, Liao Y, Huang L, Yang G, Li J (2017) The impacts of single nucleotide polymorphisms in genes of cell cycle and NF-kB pathways on the efficacy and acute toxicities of radiotherapy in patients with nasopharyngeal carcinoma. Oncotarget 8(15):25334–25344
Ma WL, Liu R, Huang LH, Zou C, Huang J, Wang J, Chen SJ, Meng XG, Yang JK, Li H, Yang GP, Guo CX (2017) Impact of polymorphisms in angiogenesis-related genes on clinical outcomes of radiotherapy in patients with nasopharyngeal carcinoma. Clin Exp Pharmacol Physiol 44(5):539–548
Tan J, Jiang L, Cheng X, Wang C, Chen J, Huang X, Xie P, Xia D, Wang R, Zhang Y (2017) Association between VEGF-460T/C gene polymorphism and clinical outcomes of nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Onco Targets Ther 10(2):909–918
Tse KP, Tsang NM, Chen KD, Li HP, Liang Y, Hsueh C, Chang KP, Yu JS, Hao SP, Hsieh LL, Chang YS (2007) MCP-1 promoter polymorphism at 2518 is associated with metastasis of nasopharyngeal carcinoma after treatment. Clin Cancer Res 13(21):6320–6326
Ghandri N, Gabbouj S, Farhat K, Bouaouina N, Abdelaziz H, Nouri A, Chouchane L, Hassen E (2011) Association of HLA-G polymorphisms with nasopharyngeal carcinoma risk and clinical outcome. Hum Immunol 72(2):150–158
Zhang YW, Ding LS, Lai MD (2003) Reg gene family and human diseases. World J Gastroenterol 9(12):2635–2641
Carrere J, Guy-Crotte O, Gaia E, Figarella C (1999) Immunoreactive pancreatic Reg protein insera from cystic fibrosis patients with and without pancreatic in sufficiency. Gut 44(4):545–551
Fukui H, Fujii S, Takeda J, Kayahara T, Sekikawa A, Nanakin A, Suzuki K, Hisatsune H, Seno H, Sawada M, Fujimori T, Chiba T (2004) Expression of RegIa protein in human gastric cancers. Digestion 69(3):177–184
Rechreche H, Montalto G, Mallo GV, Vasseur S, Marasa L, Soubeyran P, Dagorn JC, Iovanna JL (1999) pap, regIa and regIb mRNAs are concomitantly up-regulated during human colorectal carcinogenesis. Int J Cancer 81(5):688–694
Zhou L, Zhang R, Wang L, Shen S, Okamoto H, Sugawara A, Xia L, Wang X, Noguchi N, Yoshikawa T, Uruno A, Yao W, Yuan Y (2010) Upregulation of REG I alpha accelerates tumor progression in pancreatic cancer with diabetes. Int J Cancer 127(8):1795–1803
Sasaki Y, Minamiya Y, Takahashi N, Nakagawa T, Katayose Y, Ito A, Saito H, Motoyama S, Ogawa J (2008) REG1A expression is an independent factor predictive of poor prognosis in patients with breast cancer. Ann Surg Oncol 15(11):3244–3251
Minamiya Y, Kawai H, Saito H, Ito M, Hosono Y, Motoyama S, Katayose Y, Takahashi N, Ogawa J (2008) REG1A expression is an independent factor predictive of poor prognosis in patients with non-small cell lung cancer. Lung Cancer 60(1):98–104
Geng J, Fan J, Wang P, Fang ZJ, Xia GW, Jiang HW, Chen G, Ding Q (2009) REG1A predicts recurrence in stage Ta/T1 bladder cancer. Eur J Surg Oncol 35(8):852–857
Yuan RH, Jeng YM, Chen HL, Hsieh FJ, Yang CY, Lee PH, Hsu HC (2005) Opposite roles of human pancreatitis-associated protein and REG1A expression in hepatocellular carcinoma: association of pancreatitis-associated protein expression with low-stage hepatocellular carcinoma, beta-catenin mutation, and favorable prognosis. Clin Cancer Res 11(7):2568–2575
Aboshanif M, Kawasaki Y, Omori Y, Suzuki S, Honda K, Motoyama S, Ishikawa K (2016) Prognostic role of regenerating gene-I in patients with stage-IV head and neck squamous cell carcinoma. Diagn Pathol 11(1):79
Hayashi K, Motoyama S, Sugiyama T, Izumi J, Anbai A, Nanjo H, Watanabe H, Maruyama K, Minamiya Y, Koyota S, Koizumi Y, Takasawa S, Murata K, Ogawa J (2008) REGIalpha is a reliable marker of chemoradiosensitivity in squamous cell esophageal cancer patients. Ann Surg Oncol 15(4):1224–1231
Xing H, Chen X, Han Y (2017) Role of regenerating gene IA expression on local invasion and survival in nasopharyngeal carcinoma. Biol Res 50:37. https://doi.org/10.1186/s40659-017-0142-7
Hawrami K, Mohan V, Bone A, Hitman GA (1997) Analysis of islet regenerating (reg) gene polymorphisms in fibrocalculous pancreatic diabetes. Pancreas 14(2):122–125
Mahurkar S, Bhaskar S, Reddy DN, Rao GV, Chandak GR (2007) Comprehensive screening for reg1alpha gene rules out association with tropical calcific pancreatitis. World J Gastroenterol 13(44):5938–5943
Ryan BM, Robles AI, Harris CC (2010) Genetic variation in microRNA net-works: the implications for cancer research. Nat Rev Cancer 10(6):389–402
Acknowledgements
We are grateful to the patients and their families for participating in this study. We also thank all the clinicians, pathologists and study coordinators for their contributions to the work. We are grateful to Hongli Jiang (Ubiolab Genetics Technology Ltd. Beijing) for help in sequencing the DNA samples.
Funding
This work was supported by grants from the ShenZhen Science and Technology Innovation Fund Project and Nature Science Foundation from the Sci-Tech Department of Hainan Province (#JCYJ20160428174605066 and #JCYJ20170302153015013) and NSF-HN (#2010310162).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Institutional Ethics Committee of Chinese Science Academy University, Shenzhen Hospital approved this study.
Informed consent
Informed consent was obtained from all the individuals included in this study before pretreatment tumor tissue specimen was attained from NPC subject and healthy control.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xing, H., Chen, X., Sun, H. et al. Association of regenerating gene 1A single-nucleotide polymorphisms and nasopharyngeal carcinoma susceptibility in southern Chinese population. Eur Arch Otorhinolaryngol 277, 221–226 (2020). https://doi.org/10.1007/s00405-019-05645-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05645-9