Abstract
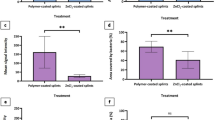
Infection is a serious complication after nasal packing that otolaryngologists seek to avoid. The aim of this study is to investigate the use of silver (Ag) nanoparticle, which serves as antimicrobial agents, with nasal tampons. The study design is an experimental animal model and the setting is tertiary referral center. Twenty-four rats were randomized into the following four groups: (1) control group (n = 6); (2) silicone nasal splint (SNS) group (n = 6); (3) polypropylene-grafted polyethylene glycol (PP-g-PEG) amphiphilic graft copolymer-coated SNS group (n = 6); and (4) Ag nanoparticle-embedded PP-g-PEG (Ag-PP-g-PEG) amphiphilic graft copolymer-coated SNS group (n = 6). These tampons were applied to rats for 48 h, after which they were removed in a sterile manner, and the rats were sacrificed. The nasal septa of the rats were excised, and assessments of tissue changes in the nasal mucosa were compared among the groups. The removed tampons were microbiologically examined, and quantitative analyses were made. When the groups were compared microbiologically, there were no significant differences in bacterial colonization rates of coagulase-negative Staphylococcus spp. among the three groups (p = 0.519), but there was a statistically significant difference among bacterial colonization rates of Heamophilus parainfluenzae and Corynebacterium spp. (p = 0.018, p = 0.004). We found that H. parainfluenzae grew less robustly in the Ag-PP-g-PEG than the PP-g-PEG group (p = 0.017). However, we found no significant difference between the Ag-PP-g-PEG and SNS groups, or between the SNS and PP-g-PEG groups. The growth of Corynebacterium spp. did not differ significantly between the Ag-PP-g-PEG and SNS groups (p = 1.000). When Group 4 was compared with Group 2, the former showed less inflammation. Compared with other tampons, Ag-PP-g-PEG amphiphilic graft copolymer-coated silicone nasal tampons caused less microbiological colonization and inflammation. Therefore, the use of these tampons may prevent secondary infections and reduce the risk of developing complications by minimizing tissue damage.


Similar content being viewed by others
References
Korkut AY, Teker AM, Eren SB, Gedikli O, Askiner O (2010) A randomised prospective trial of trans-septal suturing using a novel device versus nasal packing for septoplasty. Rhinology 48:179–182
Ardehali MM, Bastaninejad S (2009) Use of nasal packs and intranasal septal splints following septoplasty. Int J Oral Maxillofac Surg 38:1022–1024
Cayonu M, Acar A, Horasanlı E, Altundag A, Salihoglu M (2014) Comparison of totally occlusive nasal pack, internal nasal splint, and transseptal suture technique after septoplasty in terms of immediate respiratory distress related to anesthesia and surgical complications. Acta Otolaryngol 13:390–394
Tan M, Kalcioglu MT, Sahin N, Bayindir T, Samdanci E, Filiz A (2015) Assessment of mucosal changes associated with nasal splint in a rabbit model. Braz J Otorhinolaryngol 81:184–189
Wang Y, Chen S, Chen J, Zhang W, Gong G, Zhou T, Kong W (2013) Bacterial biofilm formation after nasal packing in nasal mucosa-wounded mice. Am J Rhinol Allergy 27:91–95
Şereflican M, Yurttaş V, Oral M, Yılmaz B, Dağlı M (2015) Is middle ear pressure effected by nasal packings after septoplasty? J Int Adv Otol 11:63–65
Yilmaz MS, Guven M, Buyukarslan DG, Kaymaz R, Erkorkmaz U (2012) Do silicone nasal septal splints with integral airway reduce postoperative eustachian tube dysfunction? Otolaryngol Head Neck Surg 146:141–145
Schweitzer DH, Moffie BG, van der Mey AG, Thompson J (1990) A patient with toxic shock syndrome following correction of the nasal septum. Ned Tijdschr Geneeskd 134:2146–2148
Nahass RG, Gocke DJ (1988) Toxic shock syndrome associated with use of a nasal tampon. Am J Med 84:629–631
Ahmed Q, Gupta N, Kumar A, Nimesh S (2016) Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif Cells Nanomed Biotechnol 9:1–9
Balcı M, Allı A, Hazer B, Güven O, Cavicchi K, Çakmak M (2010) Synthesis and characterization of novel comb-type amphiphilic graft copolymers containing polypropylene and polyethylene glycol. Polym Bull 64:691–705
Kalaycı ÖA, Cömert FB, Hazer B, Atalay T, Cavicchi K, Çakmak M (2010) Synthesis, characterization, and antibacterial activity of metal nanoparticles embedded into amphiphilic comb-type graft copolymers. Polym Bull 65:215–226
Garcia LS, Isenberg HD (2010) Wound cultures. In: Clinical microbiology procedures handbook. 3rd edn. ASM Press, Washington, DC, pp 441–462
Jallah Z, Liang R, Feola A, Barone W, Palcsey S, Abramowitch SD, Yoshimura N, Moalli P (2016) The impact of prolapse mesh on vaginal smooth muscle structure and function. BJOG 123:1076–1085
Plencner M, Prosecká E, Rampichová M, East B, Buzgo M, Vysloužilová L, Hoch J, Amler E (2015) Significant improvement of biocompatibility of polypropylene mesh for incisional hernia repair by using poly-ε-caprolactone nanofibers functionalized with thrombocyte-rich solution. Int J Nanomed 10:2635–2646
Chue WL, Campbell GR, Caplice N, Muhammed A, Berry CL, Thomas AC, Bennett MB, Campbell JH (2004) Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular grafts. J Vasc Surg 39:859–867
Gogoi D, Choudhury AJ, Chutia J, Pal AR, Khan M, Choudhury M, Pathak P, Das G, Patil DS (2014) Development of advanced antimicrobial and sterilized plasma polypropylene grafted muga (Antheraea assama) silk as suture biomaterial. Biopolymers 101:355–365
Scheidbach H, Tamme C, Tannapfel A, Lippert H, Köckerling F (2003) In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pig. Surg Endosc 18:211–220
Harris JM (ed) (1992) Poly(ethylene glycol) chemistry: biotechnical and biomedical applications. Plenum Press, New York
Li X, Loh XJ, Li J (2005) Poly (ester urethane) s consisting of poly [(R)-3-hydroxybutyrate] and poly(ethylene glycol) as candidate biomaterials: characterization and mechanical property study. Biomacromolecules 6:2740–2747
Markowska K, Grudniak AM, Wolska KI (2013) Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol 60:523–530
Rai M, Kon K, Ingle A et al (2014) Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol 98:1951–1961
Hazer DB, Mut M, Dincer N et al (2012) The efficacy of silver-embedded polypropylene-grafted polyethylene glycol-coated ventricular catheters on prevention of shunt catheter infection in rats. Childs Nerv Syst 28:839–846
Nair LS, Laurencin CT (2008) Nanofibers and nanoparticles for orthopedic surgery applications. J Bone Jt Surg Am 90:128–131
Savolainen S, Ylikoski J, Jousimies-Somer H (1986) The bacterial flora of the nasal cavity in healthy young men. Rhinology 24:249–255
Hazer DB, Sakar M, Dere Y, Altinkanat G, Ziyal MI, Hazer B (2016) Antimicrobial effect of polymer-based silver nanoparticle coated pedicle screws: experimental research on biofilm inhibition in rabbits. Spine (Phila Pa 1976) 41:323–329
Middleton AM, Dowling RB, Mitchell JL, Watanabe S, Rutman A, Pritchard K, Tillotson G, Hill SL, Wilson R (2003) Haemophilus parainfluenzae infection of respiratory mucosa. Respir Med 97:375–381
Wald ER (1988) Sinusitis in children. Pediatr Infect Dis J 7:150–153
Biswas K, Hoggard M, Jain R, Taylor MW, Douglas RG (2015) The nasal microbiota in health and disease: variation within and between subjects. Front Microbiol 9:134
Liu Q, Lu X, Bo M, Qing H, Wang X, Zhang L (2014) The microbiology of chronic rhinosinusitis with and without nasal polyps. Acta Otolaryngol 134:1251–1258
Kim RJ, Biswas K, Hoggard M, Taylor MW, Douglas RG (2015) Paired analysis of the microbiota of surface mucus and whole-tissue specimens in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2(5):877–883
Acknowledgements
We would to thank Assistant Professor Fürüzan Köktürk, from the Biostatistics department of Bülent Ecevit University Faculty of Medicine, for performing the statistical analyses in this study; and Nilüfer Ugur Özlük, from the Microbiology department of Bülent Ecevit University Faculty of Medicine, for the microbiological analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Funding
This study was not funded.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Şevik Eliçora, S., Erdem, D., Dinç, A.E. et al. Effects of polymer-based, silver nanoparticle-coated silicone splints on the nasal mucosa of rats. Eur Arch Otorhinolaryngol 274, 1535–1541 (2017). https://doi.org/10.1007/s00405-016-4394-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4394-6