Abstract
Purpose
This study aimed at investigating the associations between the total body mass index (BMI) change at 3 or 4 years postpartum compared to the prepregnancy and cardiometabolic risk factors.
Methods
This longitudinal study included 1305 participants. Based on the total postpartum BMI changes, they were divided into < 0 units, 0–1.7 units, and > 1.7 units groups using the interquartile range. Multiple linear regression models were used to analyze the associations.
Results
Compared to the reference group, there was a progressive increase in the βcoefficient (βcoef) of homeostasis model assessment of insulin resistance (HOMA-IR) of cardiometabolic risk in the following groups: the ‘0–1.7 units’ group with the ‘overweight traj’ [βcoef 0.33; 95% confidence intervals (CI) 0.22, 0.44)] or the ‘obesity traj’ [0.66; (0.45, 0.88)] and the ‘> 1.7 units’ group with the ‘normal traj’ [0.33; (0.22, 0.44)], the ‘overweight traj’ [0.54; (0.41, 0.67)] or the ‘obesity traj’ [0.97; (0.79, 1.15)]. The same increasing trend of βcoef was also found in DBP, FPG, LDL, WHR, BF%. However, the ‘< 0 units’ group with the ‘low traj’ [0.13; (0.06, 0.21)] and the ‘0–1.7 units’ group with the ‘low traj’ [0.08; (0.03, 0.13)] had higher high-density lipoprotein cholesterol (HDL-C) level than the reference group.
Conclusion
Women with a postpartum BMI gain > 1.7 units are positively associated with cardiometabolic risk factors, especially for those in the ‘obesity traj’ or ‘traj D’. Conversely, women with a postpartum BMI loss > 0 units have negative association with cardiometabolic risk factors, especially for those in the ‘low traj’ or ‘traj B’.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
To further investigate the correlation between BMI changes from the prepregnancy to postpartum period and cardiometabolic risk factors, taking into account the effects of prepregnancy BMI and gestational weight gain (GWG). In clinical terms, it gives Chinese women a reference for maternal weight recovery after delivery. |
Introduction
In 2014, China became the country with the highest number of women who are slightly obese worldwide [1]. And even for severe obesity, the number of Chinese women got to 2nd rank [1]. Obesity, which negatively impacts women’s health, has much wider and long-lasting consequences and is generally taken seriously by either health professionals or women themselves [2]. For reproductive-aged women, one of the risk factors for obesity is pregnancy [3,4,5], possibly through the mechanism of postpartum weight retention or weight gain [6,7,8,9]. Cardiometabolic risk is the leading contributor to the disease burden for Chinese women [10]. Obesity is a modifiable risk factor of cardiovascular disease [11], and more than two-thirds of deaths related to high body mass index (BMI) are due to cardiovascular disease [12]. Moreover, postpartum weight gain may cause abdominal adiposity in some women [13], which increases the risk of cardiovascular and metabolic diseases [13, 14]. Therefore, research on the relationship between maternal BMI changes from the prepregnancy to postpartum period and cardiometabolic risk is of great significance to Chinese women’s health.
Similarly, considering both prepregnancy BMI and excessive gestational weight gain (GWG) as risk factors for postpartum weight retention or weight gain [6, 8, 9, 15], we also evaluated their effects on the total postpartum BMI changes. In contrast to the classification criteria used in previous studies: international prepregnancy BMI classification, Institute of Medicine's GWG-recommended classification [16], or self-classified GWG classification [15], we dynamically account for changes in weight from prepregnancy through pregnancy in the form of trajectories. Because even if women have the same prepregnancy BMI or GWG, there might be different subgroups, and gestational weight is always in a dynamic change due to changeable pregnancy situation, which may potentially affect the postpartum weight, and then affects assessing cardiometabolic risk. To date, there are limited studies that can simultaneously account for prepregnancy BMI, GWG, postpartum weight changes, and postpartum cardiovascular risk [16, 17]. To the best of our knowledge, no studies have explored postpartum BMI changes considering the effects of the prepregnancy-to-pregnancy weight trajectory and postpartum cardiometabolic risk factors. Thus, the current study aimed to answer this question.
Methods
Study population
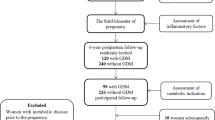
We conducted a longitudinal study included 1346 pregnant women aged ≥ 18 years who delivered a full-term live neonate at Shenzhen Maternity & Child Healthcare Hospital, a government hospital in the fourth largest city of China, between 2016 and 2018 and had weight data for the prepregnancy period and the first, second, and third trimesters. A follow-up study was conducted between 2020 and 2021, that is, at 3 or 4 years postpartum, to collect data on basic information and outcome variables. We excluded women with missing data on postpartum BMI (n = 5, 0.37%) and covariates (n = 36, 2.67%). Ultimately, 1305 women were included in the present study (Fig. 1). The Shenzhen Maternity & Child Healthcare Hospital Research Ethics Board approved the study protocol, and all participants signed informed consent forms.
Exposure variables
Total BMI change from the prepregnancy to postpartum period
We calculated the participants’ difference of BMI [weight (kg)/height (m2)] units between the postpartum and prepregnancy and divided them into three groups using the interquartile range (IQR)as follows: the postpartum BMI loss group: < 0 units; the reference group: the first quantile to the third quartile, or 0–1.7 units; and the postpartum BMI gain group: > 1.7 units. Prepregnancy BMI was obtained from the hospital medical system. Postpartum BMI was measured by trained research assistants or nurses at the 3- or 4-year postpartum interview.
Prepregnancy-to-pregnancy BMI trajectories
We chose four points to model BMI trajectories, including the prepregnancy BMI, first-trimester BMI, second-trimester BMI, and third-trimester BMI. Considering that pregnant women visited in different weeks during every trimester, we believed that as long as there was one BMI data for the first (week 10 to week 13), second (week 24 to week 27) and third (week 36 to week 39) trimesters, it could be regarded as the BMI of the period.
Outcome variables
Cardiometabolic risk factors at 3 or 4 years postpartum
At the 3-year or 4-year postpartum visit, the waist-to-hip ratio (WHR) and body fat percentage (BF%) were measured using a TSINGHUA TONGFANG BCA-1B. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured 2 times, 2 min apart, with an OMRON HBP-9020; then, the average SBP and DBP were calculated and were included in the final models. Blood was collected to measure biochemical data. Fasting plasma glucose (FPG) was assayed by a hexokinase/glucose-6-phosphate dehydrogenase method, and fasting insulin was measured with a microparticle enzyme immunoassay. Then, the homeostasis model assessment of insulin resistance (HOMA-IR) [fasting insulin (µU/mL) × fasting plasma glucose (mmol/L)/22.5] was calculated. The triglyceride (TG) and total cholesterol (TC) levels were assayed by enzymatic methods. Low-density lipoprotein cholesterol (LDL-C) level was measured with a homogenous direct method, and high-density lipoprotein cholesterol (HDL-C) level was measured using a direct enzymatic colorimetric assay. Since carotid intima-media thickness (CIMT) was shown to predict cardiovascular risk in multiple large studies [18, 19] and associated with cardiovascular disease in Asians [20], we also included the maximum CIMT (CIMT max) measured using a Phillips-SD 800 7.5 MHz transducer as a cardiometabolic risk factor. All of the above data were measured using the standardized protocol by trained research assistants or nurses.
Covariates
The women’s sociodemographic information, including age, education level, and parity, was obtained. Information on diabetes, gestational diabetes mellitus and pregestational diabetes mellitus, hypertensive disorders of pregnancy, and infant’s gender was obtained from the hospital medical system. Information on smoking habits and alcohol intake was also provided but did not alter the βcoefficients (βcoef) or 95% confidence intervals (CI) and were not included in the final models.
Statistical analysis
We used 4 points as mentioned above to identify prepregnancy-to-pregnancy BMI trajectories using latent class growth modelling (LCGM) [21]. LCGM, an example of finite mixture models, is a method of modeling developmental trajectories that classify individuals within a population that fall into specific clusters. The estimation of the model parameters is achieved by maximum likelihood. The shape and number of the final trajectory groups are based on five aspects, including a priori knowledge, the Bayesian information criterion (BIC), choice of polynomial terms, the average posterior probability (≥ 0.70 for each group), and parsimony in the number of trajectory groups. See “Traj” package in STATA for more details. Ultimately, we obtained 4 trajectories and named them from bottom to top as follows: the ‘low traj’, ‘normal traj’, ‘overweight traj’, ‘obesity traj’.
To estimate the associations between the total BMI change and cardiometabolic risk factors at 3 or 4 years postpartum, we used multiple linear regression (MLR) models to estimate the βcoef and 95% CI. To explore how gestational BMI trajectory groups modify the association between the total BMI change and cardiometabolic risk factors, we evaluated how permutations of the gestational BMI trajectory groups and the total BMI change in 12 groups were associated with cardiometabolic risk factors while simultaneously considering Bonferroni’s correction (corrected p < 0.005). Women in the ‘0–1.7 units’ group with the ‘normal traj’ served as the reference group. The models were adjusted for sociodemographic information, diabetes, hypertensive disorders of pregnancy, and infant’s gender.
Considering that women with different prepregnancy BMI is recommended for corresponding GWG, there may be underlying trend trajectories. To eliminate this potential effect, we chose the group of women with a normal prepregnancy BMI and then set up a permutation of the trajectories and the total BMI change for further MLR with cardiometabolic risk factors.
All analyses were conducted in Stata MP (version 16).
Results
Table 1 summarizes the sociodemographic, obstetrical and follow-up information of the study participants. These characteristics of the women were comparable among each total BMI change group. The women were of a mean age of 31.9 years at enrollment, and most of them had a college education (78.3%), had a normal prepregnancy BMI (70.1%), had given birth only once (56.4%), and had no hypertensive disorders of pregnancy (98.8%) or diabetes during pregnancy (80.7%). The means of the postpartum physical examination results were all normal. Women in the ‘> 1.7 units’ group (mean age 31.3 years) were younger and accounted for 24.5% of the sample, and women in the ‘< 0 units’ group (mean age 32.1 years) were older and accounted for 24.1% of the sample compared with the ‘0–1.7 units’ group (mean age 32.0 years).
Prepregnancy-to-pregnancy BMI trajectories
The four trajectories are shown in Fig. 2. In mid-pregnancy, the rate of gestational BMI gain was significantly faster compared with that in early pregnancy, and the rates of gestational BMI gain in the first three trajectories slowed in late pregnancy according to final polynomial terms. The first three trajectories included 28.4%, 39.4% and 25.6% of the women, respectively. Only the ‘obesity traj’ maintained a constant weight gain rate from mid-pregnancy onward and included 6.6% of the women. Furthermore, the starting points of the four trajectories, the prepregnancy BMI, were 18.57, 21.08, 23.93, 28.47, and it seemed to be consistent with the BMI of Chinese women (low: < 18.5, normal: 18.5–23.9, overweight: 24–27.9, obesity: > 28) [24]. So we named the four trajectories from bottom to top as the ‘low traj’, ‘normal traj’, ‘overweight traj’, ‘obesity traj’, and ‘normal traj’ served as reference.
Associations between the total BMI change and cardiometabolic risk factors
Table 2 shows the associations between the total BMI change from the prepregnancy to postpartum period and cardiometabolic risk factors stratified by BMI trajectory groups. Compared to the reference group, there was a progressive increase in the βcoef of HOMA-IR of cardiometabolic risk in the following groups: the ‘0–1.7 units’ group with the ‘overweight traj’ [βcoef 0.33; 95% CI 0.22, 0.44)] or the ‘obesity traj’ [0.66; (0.45, 0.88)] and the ‘> 1.7 units’ group with the ‘normal traj’ [0.33; (0.22, 0.44)], the ‘overweight traj’ [0.54; (0.41, 0.67)] or the ‘obesity traj’ [0.97; (0.79, 1.15)] in the postpartum period. The same increasing trend of βcoef was also found in DBP, FPG, LDL, WHR, BF%. However, the ‘< 0 units’ group with the ‘low traj’ [0.13; (0.06, 0.21)] and the ‘0–1.7 units’ group with the ‘low traj’ [0.08; (0.03, 0.13)] had higher HDL-C level than the reference group. These two groups also had lower WHR and BF% values than the reference group. For adiposity factors, the WHR and BF% were statistically significant in almost all groups. The ‘0–1.7 units’ group with the ‘obesity traj’ had a higher CIMT max [0.06 (0.03, 0.10)] than the reference group.
Associations between the total BMI change and cardiometabolic health in women with normal prepregnancy BMI
The four trajectories trends shown in Fig. 2 appear to be influenced by prepregnancy BMI, so we selected women with normal prepregnancy BMI to construct further trajectories and then analyze the relationships between these trajectories and cardiometabolic risk factors. The four trajectories are shown in Fig. 3 and had similar trends as those shown in Fig. 2. The four trajectories included 12.5%, 33.4%, 37.6% and 16.5% of the women respectively. It can be seen from the figure that the rate of gestational BMI gain (traj A: 19.33–22.82; traj B: 19.99–25.36; traj C: 21.68–27.38; traj D: 22.93–29.47) gradually accelerates with the increase of the trajectories. And according to prepregnancy BMI and the rate of gestational BMI gain, we named the four trajectories from bottom to top as follows: the traj A (very low rate of gestational BMI gain traj), traj B (low rate of gestational BMI gain traj), traj C (normal rate of gestational BMI gain traj), traj D (high rate of gestational BMI gain traj). The ‘traj C’ served as the reference group.
The MLR results in Table 3 showed the same direction in further analysis. And women in the ‘0–1.7 units’ group with the ‘traj C’ served as the reference group.
Discussion
Based on the results of this longitudinal study, we observed a positive association between the ‘0–1.7 units’ group or ‘> 1.7 units’ group with the ‘normal traj’ or the ‘overweight traj’ or the ‘obesity traj’ and cardiometabolic risk factors. The opposite was found in the ‘< 0 units’ group or ‘0–1.7 units’ group with the ‘low traj’. These associations were consistent in our further MLR analysis among women who had a normal prepregnancy BMI.
Pregnancy is a risk factor for obesity, especially abdominal adiposity, and these physiological alterations may increase cardiometabolic risk when they extend beyond pregnancy and the traditional postpartum period [25,26,27,28,29,30,31,32]. This is consistent with our findings that the higher the postpartum weight is, the higher the WHR and BF%. It has been speculated that postpregnancy obesity may in part contribute to fertility-related low HDL-C levels [25, 27, 28]. We also found the same results. Diana et al. also used LCGM to model weight trajectories during the two years postpartum and then analyzed the association between the weight trajectories and cardiometabolic markers, concluding that women in the little weight loss + the slight gain trajectory group were heavier and had higher central obesity, inflammation and LDL-C levels at 3 years postpartum [17]. This corresponded to our postpartum BMI gain ‘> 1.7 units’ group, and we also observed that this group had central obesity and high LDL-C level. In a large cohort study with 16 years of follow-up, participants were divided into 4 groups based on the total BMI change from the prepregnancy to postpartum period: < − 1, − 1 to 1, > 1 to 2, and > 2 [16]. An increased risk of hypertension and CVD with postpartum weight gain was observed [16]. Considering low HDL-C level and HOMA-IR as two of the cardiovascular risk factors, we obtained that postpartum BMI gain > 1.7 units was associated with low HDL-C level and high HOMA-IR, and thus we have a possible explanation that postpartum BMI gain increased potential cardiometabolic risk. Hayfaa et al. categorized participants into different levels according to their postpartum weight retention (weight retention < 3 kg; weight retention 3 to < 7 kg; and weight retention ≥ 7 kg) to explore the association between different weight retention levels at 12 months postpartum and cardiometabolic risk [33]. They concluded that cardiometabolic risk augmented with the increase of postpartum weight [33]. Taking into account ethnicity differences, our study found a positive association between postpartum BMI gain > 1.7 units and cardiometabolic risk factors. In addition, other studies also characterized postpartum weight in different ways at 12 months postpartum to prove the association with cardiometabolic risk factors [34, 35]. A follow-up study over 15 years found women who failed to lose weight over 4 years postpartum to be in a significant risk of obesity in midlife [36]. Our study also showed that postpartum BMI gain > 1.7 units at 3 or 4 years postpartum was strongly associated with high WHR and BF%. Furthermore, Lorenz et al. indicated that every 0.1 mm increase in the CIMT was associated with a 10–15% increase in the risk of future myocardial infarction [37]. In our study, we observed that the ‘0–1.7 units’ group with the ‘obesity traj’ had a higher CIMT than the reference group.
Unlike the previously described associations between postpartum weight gain and cardiometabolic risk factors, Diana et al. observed the opposite result as follows: women in fast weight loss + slight gain group developed less weight, less central adiposity and lower insulin resistance [17]. The corresponding finding that WHR and BF% decreased with decreasing postpartum weight in the ‘low traj’ was also observed in our study. We also observed high HDL-C level, which is a strong protective factor against CVD [38], in these groups. A nested comparative study investigating how postpartum weight retention affects ox-LDL and serum lipids stratified participants into tertiles, including weight loss, unaltered weight and weight gain groups. They reported the same results, with women who lost weight having lower TG and ox-LDL/HDL-C level ratios than women who gained weight [39]. Discussing the relationships among three weight loss interventions and cardiometabolic risk factors, including blood pressure, lipids, glucose and insulin resistance, an investigation revealed that enhanced brief lifestyle counseling, which led to the most weight loss, was associated with the largest improvements in HDL-C level and TG levels [40]. Furthermore, other studies found that postpartum weight loss appeared to be associated with the improvement of cardiometabolic risk factors [41,42,43,44,45].
Strengths and limitations
The major strength of this study is that it is the first large population-based study on the association between the total BMI change from the prepregnancy to postpartum period and postpartum cardiometabolic risk factors in Chinese. This study is essential and informative, as the number of women with obesity in China is increasing with socioeconomic development; the cardiovascular risks involved are also increasing [2, 10, 46]. Another advantage is that to prevent potential established trends in all women, further analysis was performed in participants with normal prepregnancy BMI. Furthermore, the acquisition of data on our exposure and outcome variables was performed by trained and experienced nurses or assistants, which reduced the risk of bias. Our study on cardimetabolic risk factors also applied noninvasive ultrasonography CIMT, which may be a screening method in the future.
There are also some limitations in our study. The major limitation of this study is that because it was a longitudinal study on cardiometabolic risk factors, causality cannot be inferred in the final association between the total BMI change and cardiovascular and metabolic disease. However, we aimed to study a relatively short period of 3 or 4 years postpartum to guide women manage their postpartum weight, which would also reduce to some extent the possible occurrence of cardiovascular disease afterwards. Further, It would be of great interest to perform a prospective follow-up on the occurrence of endpoint events. Although we adjusted for several potential confounders, we could not exclude residual or other unmeasured confounders. Despite these limitations, our data were sufficient to examine the association between the total BMI change from the prepregnancy to postpartum period and cardiometabolic risk factors.
Conclusion
In conclusion, postpartum BMI gain > 1.7 units is associated with cardiometabolic risk factors, especially for women in the ‘obesity traj’. However, postpartum weight loss > 0 units compared to the prepregnancy weight will contribute to improving cardiometabolic outcomes. Our findings therefore underscore the importance of postpartum weight loss interventions for improving women's health.
Data availability
Due to the participants of this study did not agree for their data to be shared publicly.
References
(2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387(10026):1377-1396. https://doi.org/10.1016/S0140-6736(16)30054-X
Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, Gillman MW (2016) Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 4(12):1025–1036. https://doi.org/10.1016/S2213-8587(16)30217-0
Gunderson EP, Abrams B (2000) Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev 22(2):261–274. https://doi.org/10.1093/oxfordjournals.epirev.a018038
Phelan S (2010) Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol 202(2):131–135. https://doi.org/10.1016/j.ajog.2009.06.008
Endres LK, Straub H, Mckinney C, Plunkett B, Minkovitz CS, Schetter CD, Ramey S, Wang C, Hobel C, Raju T, Shalowitz MU (2015) Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol 125(1):144–152. https://doi.org/10.1097/AOG.0000000000000565
Gore SA, Brown DM, West DS (2003) The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med 26(2):149–159. https://doi.org/10.1207/S15324796ABM2602_07
Olson CM (2007) A call for intervention in pregnancy to prevent maternal and child obesity. Am J Prev Med 33(5):435–436. https://doi.org/10.1016/j.amepre.2007.07.028
Shao HH, Hwang LC, Huang JP, Hsu HY (2018) Postpartum weight retention risk factors in a Taiwanese cohort study. Obes Facts 11(1):37–45. https://doi.org/10.1159/000484934
Hill B, Mcphie S, Skouteris H (2016) The role of parity in gestational weight gain and postpartum weight retention. Women Health Issues 26(1):123–129. https://doi.org/10.1016/j.whi.2015.09.012
(2016) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1659–1724. https://doi.org/10.1016/S0140-6736(16)31679-8
Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J (2011) Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 377(9771):1085–1095. https://doi.org/10.1016/S0140-6736(11)60105-0
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, Mcalinden C, Mcgarvey ST, Mengistu DT, Mensah GA, Mensink G, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva D, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray C (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377(1):13–27. https://doi.org/10.1056/NEJMoa1614362
Mcclure CK, Catov JM, Ness R, Bodnar LM (2013) Associations between gestational weight gain and BMI, abdominal adiposity, and traditional measures of cardiometabolic risk in mothers 8 y postpartum. Am J Clin Nutr 98(5):1218–1225. https://doi.org/10.3945/ajcn.112.055772
Haffner SM (2007) Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med 120(9 Suppl 1):S10–S17. https://doi.org/10.1016/j.amjmed.2007.06.006
Turner MJ, Layte R (2013) Obesity levels in a national cohort of women 9 months after delivery. Am J Obstet Gynecol 209(2):121–124. https://doi.org/10.1016/j.ajog.2013.04.020
Kirkegaard H, Bliddal M, Støvring H, Rasmussen KM, Gunderson EP, Køber L, Sørensen T, Nøhr EA (2021) Maternal weight change from prepregnancy to 18 months postpartum and subsequent risk of hypertension and cardiovascular disease in Danish women: a cohort study. Plos Med 18(4):e1003486. https://doi.org/10.1371/journal.pmed.1003486
Soria-Contreras DC, Rifas-Shiman SL, Aris IM, Perng W, Switkowski KM, Téllez-Rojo MM, Trejo-Valdivia B, López-Ridaura R, Oken E (2020) Weight trajectories after delivery are associated with adiposity and cardiometabolic markers at 3 years postpartum among women in project viva. J Nutr 150(7):1889–1898. https://doi.org/10.1093/jn/nxaa104
Naqvi TZ, Lee MS (2014) Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 7(10):1025–1038. https://doi.org/10.1016/j.jcmg.2013.11.014
Polak JF, O’Leary DH (2016) Carotid intima-media thickness as surrogate for and predictor of CVD. Glob Heart 11(3):295–312. https://doi.org/10.1016/j.gheart.2016.08.006
Shimoda S, Kitamura A, Imano H, Cui R, Muraki I, Yamagishi K, Umesawa M, Sankai T, Hayama-Terada M, Kubota Y, Shimizu Y, Okada T, Kiyama M, Iso H (2020) Associations of carotid intima-media thickness and plaque heterogeneity with the risks of stroke subtypes and coronary artery disease in the japanese general population: the circulatory risk in communities study. J Am Heart Assoc 9(19):e17020. https://doi.org/10.1161/JAHA.120.017020
Jones BL, Nagin DS (2013) A note on a Stata plugin for estimating group-based trajectory models. Sociol Method Res 42(4):608–613
Flegal KM (2021) BMI and obesity trends in Chinese national survey data. Lancet 398(10294):5–7. https://doi.org/10.1016/S0140-6736(21)00892-8
(2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403): 157–163. https://doi.org/10.1016/S0140-6736(03)15268-3
The Working Group of Obesity, China (2004) Guidelines for the prevention and control of overweight and obesity in Chinese adults (excerpt). Acta Nutr Sin (01):1–4
Vladutiu CJ, Siega-Riz AM, Sotres-Alvarez D, Stuebe AM, Ni A, Tabb KM, Gallo LC, Potter JE, Heiss G (2016) Parity and components of the metabolic syndrome among Us Hispanic/Latina women: results from the Hispanic community health study/study of Latinos. Circ Cardiovasc Qual Outcomes 9(2 Suppl 1):S62–S69. https://doi.org/10.1161/CIRCOUTCOMES.115.002464
Gunderson EP, Sternfeld B, Wellons MF, Whitmer RA, Chiang V, Quesenberry CJ, Lewis CE, Sidney S (2008) Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 16(5):1078–1084. https://doi.org/10.1038/oby.2008.40
Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith WD, Sidney S (2004) Long-term plasma lipid changes associated with a first birth: the coronary artery risk development in young adults study. Am J Epidemiol 159(11):1028–1039. https://doi.org/10.1093/aje/kwh146
Kritz-Silverstein D, Barrett-Connor E, Wingard DL (1992) The relationship between multiparity and lipoprotein levels in older women. J Clin Epidemiol 45(7):761–767. https://doi.org/10.1016/0895-4356(92)90053-p
Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Gunderson EP, Stuebe AM, Mantzoros CS (2011) Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity (Silver Spring) 19(1):171–178. https://doi.org/10.1038/oby.2010.117
Groth SW, Fernandez ID, Block RC, Thurston SW, Wong E, Brunner J, Mayo N, Kapula N, Yu Y, Meng Y, Yeh KL, Kinkade CW, Thornburg LL, O’Connor TG, Barrett ES (2021) Biological changes in the pregnancy-postpartum period and subsequent cardiometabolic risk-UPSIDE MOMS: a research protocol. Res Nurs Health 44(4):608–619. https://doi.org/10.1002/nur.22141
Nicklas JM, Leiferman JA, Lockhart S, Daly KM, Bull SS, Barbour LA (2020) Development and modification of a mobile health program to promote postpartum weight loss in women at elevated risk for cardiometabolic disease: single-arm pilot study. JMIR Form Res 4(4):e16151. https://doi.org/10.2196/16151
Linné Y, Dye L, Barkeling B, Rössner S (2004) Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res 12(7):1166–1178. https://doi.org/10.1038/oby.2004.146
Wahabi HA, Fayed AA, Tharkar S, Esmaeil SA, Bakhsh H (2019) Postpartum weight retention and cardiometabolic risk among Saudi women: a follow-up study of RAHMA subcohort. Biomed Res Int 2019:2957429. https://doi.org/10.1155/2019/2957429
Soria-Contreras DC, Trejo-Valdivia B, Cantoral A, Pizano-Zárate ML, Baccarelli AA, Just AC, Colicino E, Deierlein AL, Wright RO, Oken E, Téllez-Rojo MM, López-Ridaura R (2020) Patterns of weight change one year after delivery are associated with cardiometabolic risk factors at six years postpartum in Mexican women. Nutrients 12(1):170. https://doi.org/10.3390/nu12010170
Kew S, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R (2014) Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care 37(7):1998–2006. https://doi.org/10.2337/dc14-0087
Rooney BL, Schauberger CW, Mathiason MA (2005) Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol 106(6):1349–1356. https://doi.org/10.1097/01.AOG.0000185480.09068.4a
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115(4):459–467. https://doi.org/10.1161/CIRCULATIONAHA.106.628875
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977) High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med 62(5):707–714. https://doi.org/10.1016/0002-9343(77)90874-9
Puhkala J, Luoto R, Ahotupa M, Raitanen J, Vasankari T (2013) Postpartum weight retention is associated with elevated ratio of oxidized LDL lipids to HDL-cholesterol. Lipids 48(12):1227–1235. https://doi.org/10.1007/s11745-013-3852-9
Vetter ML, Wadden TA, Chittams J, Diewald LK, Panigrahi E, Volger S, Sarwer DB, Moore RH (2013) Effect of lifestyle intervention on cardiometabolic risk factors: results of the POWER-UP trial. Int J Obes (Lond) 37(Suppl 1):S19–S24
Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, Sharma P, Fraser C, Maclennan G (2017) Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ 359:j4849. https://doi.org/10.1136/bmj.j4849
Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, Mamas MA, Loke YK (2014) Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol 173(1):20–28. https://doi.org/10.1016/j.ijcard.2014.02.026
Goldstein DJ (1992) Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 16(6):397–415
Nilsson PM (2008) Is weight loss beneficial for reduction of morbidity and mortality? What is the controversy about? Diabetes Care 31(Suppl 2):S278–S283. https://doi.org/10.2337/dc08-s268
Sjöström CD, Peltonen M, Wedel H, Sjöström L (2000) Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 36(1):20–25. https://doi.org/10.1161/01.hyp.36.1.20
Twinn SF, Lee GC, Thompson DR (2010) A survey of lifestyle risk factors for cardiovascular health among young Hong Kong Chinese women: implications for long-term cardiovascular health. J Womens Health (Larchmt) 19(2):289–295. https://doi.org/10.1089/jwh.2008.1318
Acknowledgements
The authors would like to thank all research assistants and nurses in Shenzhen Maternity & Child Healthcare Hospital for their assistance to this study. The authors thank Prof. Xin Zhou at Tianjin University General Hospital about the help on the latent class growth modelling.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81830041) and Shenzhen Science and Technology Program (JCYJ20220818103608017, JCYJ20220818103607015, JSGG20210802152800001).
Author information
Authors and Affiliations
Contributions
JN contributed to the study conception. HZ and F-YT contributed to the study design. Material preparation, data collection were performed by LW, XW, YC, AY, FH, JT, XH, YW. HZ contributed to data management and data analysis. The first draft of the manuscript was written by HZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the The Shenzhen Maternity & Child Healthcare Hospital Research Ethics Board (Shenzhen Maternal and Child Ethics Review No. 23; date of approval 7 April 2017).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, H., Wu, L., Wu, X. et al. Maternal BMI changes from the prepregnancy to postpartum period are associated with postpartum cardiometabolic risk factors: a longitudinal study. Arch Gynecol Obstet 309, 2591–2603 (2024). https://doi.org/10.1007/s00404-023-07154-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07154-x