Abstract
Purpose
Intraoperative cell salvage is central to Patient Blood Management including for lower segment caesarean section. Prior to April 2020, we initiated intraoperative cell salvage during caesarean section based on risk assessment for hemorrhage and patient factors. As the pandemic broadened, we mandated intraoperative cell salvage to prevent peri-partum anemia and potentially reduce blood product usage. We examined the association of routine intraoperative cell salvage on maternal outcomes.
Methods
We conducted a single-center non-overlapping before-after study of obstetric patients undergoing lower segment caesarean section in the 2 months prior to a change in practice (‘usual care = selective intraoperative cell salvage’, n = 203) and the 2 months following (‘mandated intraoperative cell salvage’, n = 228). Recovered blood was processed when a minimal autologous reinfusion volume of 100 ml was expected. Post-operative iron infusion and length of stay were modelled using logistic or linear regression, using inverse probability weighting to account for confounding.
Results
More emergency lower-segment caesarean sections occurred in the Usual Care group. Compared to the Usual Care group, post-operative hemoglobin was higher and anemia cases fewer in the Mandated intraoperative cell salvage group. Rates of post-partum iron infusion were significantly lower in the Mandated intraoperative cell salvage group (OR = 0.31, 95% CI = 0.12 to 0.80, P = 0.016). No difference was found for length of stay.
Conclusion
Routine cell salvage provision during lower segment caesarean section was associated with a significant reduction in post-partum iron infusions, increased post-operative hemoglobin and reduced anemia prevalence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cell salvage provision during cesarean section is a valuable tool and reduces the incidence of post-partum anemia and the need for post-partum iron infusion. It may contribute to an avoidance or reduction of allogeneic red blood cell transfusion in the peri-partum setting. |
Introduction
In March 2020, the World Health Organization (WHO) declared a pandemic status for the coronavirus disease 2019 (COVID-19). In addition to the unprecedented pressures on healthcare systems, COVID-19 has had a profound impact on the number of blood donations, leaving blood services severely challenged to maintain their inventory during the pandemic [1,2,3]. These challenges highlight the critical importance of Patient Blood Management (PBM) as an integral part of patient care. In a recent publication, a multinational and diverse group of authors issued a “Call to Action” urging all stakeholders and providers to implement practical and multimodal principles of PBM [4].
PBM is a patient-centered approach, applying an evidence-based bundle of care to preserve the patient’s own blood by managing anemia, minimizing iatrogenic blood loss, and harnessing tolerance to anemia to improve patient outcomes [5]. Intraoperative cell salvage (IOCS) is an integral and valuable component of PBM [5,6,7].
The benefits of IOCS have been known for some time [8]. Clinical adoption of IOCS in obstetrics for lower segment caesarean section (LSCS) has increased substantially, but despite this, the utilization of this technique varies considerably around the world [9,10,11]. At our institution, IOCS has been an established modality since 2013, as part of our hospital-wide PBM approach. Prior to April 2020, our institutional practice included initiation of IOCS during LSCS based on pre-operative anemia, patient risk assessment for intra-operative blood loss, post-partum hemorrhage and other individual patient factors.
In response to the Australian National Blood Authority’s call to adhere to PBM guidelines and practices during the pandemic, to preserve finite reserves of donated blood, [12, 13] members of the Departments of Anesthesia and Obstetrics at our institution discussed if modifications of our PBM practices were warranted to prevent the incidence and severity of peri-partum anemia. A decision was made to trial the mandated use of IOCS for all LSCS during the height of the first wave of the pandemic, to explore whether this practice could reduce the need for red blood cell (RBC) transfusion. We also aimed to examine the association of routine IOCS for LSCS on other indicators of optimal PBM, including the rate and volume of returned cell salvaged blood and the incidence and degree of post-partum anemia, as well as the impact on other maternal outcomes such as length of hospital stay.
Methods
This study was approved by our Institutional Review Board (IRB), (CALHN Reference Number: 14994). Written informed consent was waived by the IRB. We conducted a single-center non-overlapping before-after study of all obstetric patients undergoing either elective or emergent lower segment caesarean section over a four-month period in early 2020. Exclusion criteria were caesarean section for stillbirth or patient refusal of the utilization of cell salvage, which was discussed while obtaining anesthesia consent. This study adheres to The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement [14].
Data from selective intra-operative cell salvage practice (06 February 2020–05 April 2020) and the subsequent two months (06 April 2020–05 June 2020) with the use of routine intra-operative shed blood collection was collected from electronic and paper records, after the completion of the study period. Participants were identified via electronic hospital records, using the search terms “LSCS” and “Caesarean” between the above dates. Anemia was defined as hemoglobin (Hb) < 110 g/L during pregnancy and Hb < 100 g/L post-partum [13]. The risk of bias was low, as for both groups, the recovered blood was processed when a substantial blood loss occurred, and the autologous RBC reinfusion volume was expected to reach 100 ml or more.
Statistical analysis
Descriptive statistics summarized patient characteristics using counts and percentages for categorical data, means and standard deviations or medians and interquartile ranges for continuous data, as appropriate. To compare post-operative outcomes between the groups who have mandated IOCS or received usual care, t-tests for normally distributed continuous data, Kruskal–Wallis tests for skewed data, and chi-square tests for categorical data were used.
Post-partum iron infusion was modelled using logistic regression, and log-transformed length of stay with linear regression. Other outcomes, such as post-operative hemoglobin or anemia, were not modelled due to a large proportion of missing data. Differences between mandated IOCS and usual care were expressed as odds ratios for post-partum iron infusion and geometric mean ratios for length of stay, and 95% confidence intervals (CI). To account for potential confounding by differences in patients between time periods and types of care, a counterfactual approach with propensity modelling was used. The propensity model used mandated IOCS vs usual care as the outcome with covariates age, parity, gravida, ASA score, pre-operative Hb, operation type and anesthesia type. The propensity model was used to create trimmed, stabilized inverse probability weights (IPW). Standardized mean differences (SMD) were used to assess balance in covariates at baseline before and after weighting, where an SMD > 0.1 was considered imbalanced.
Finally, to investigate the possibility that the changes over time could have occurred regardless of mandated IOCS, an interrupted time series (ITS) analysis, adapted for short time periods, was performed using the R package ‘its.analysis’. Post-partum IV iron and length of stay were aggregated by week to create time series. A significant difference in the outcome between the two periods was tested, accounting for important covariates and autocorrelation in the outcome over time. The ITS model for a number of post-operative iron infusions per week used pre-operative anemia, operation type and primiparity as covariates, while the model for log-transformed length of stay included pre-operative Hb, emergency operation and primiparity rates.
Statistical significance was assessed at the p = 0.025 level to account for two outcomes. R statistical software version 4.1.0 (R Foundation for statistical computing, Vienna Austria) was used for all statistical analyses.
Results
A total of 431 participants were included in the study. During the first two months, 203 women underwent caesarean section delivery and received the usual care, while 228 women delivered during the period where IOCS was mandated for caesarean deliveries.
Before inverse probability weighting, 6/9 pre-partum maternal characteristics were unbalanced with SMD > 0.1 (Table 1). After weighting, the imbalance was removed (all SMD < 0.1).
There was no difference in allogeneic RBC transfusion, with only one woman in each group receiving blood products. Univariate analysis showed that post-operatively, women in the Mandated IOCS group had higher post-operative Hb values, 111 g/L vs 102 g/L (p < 0.001) (despite similar pre-operative Hb, 125 g/L vs 124 g/L), had significantly lower rates of anemia, 48% vs 78% (p < 0.001) and less often exposed to post-partum intravenous iron, 11% vs 3% (p < 0.001) (Table 2). A higher proportion of women in the Usual Care cohort had an emergency caesarean section (p = 0.03), greater estimated blood loss (EBL) (p = 0.003).
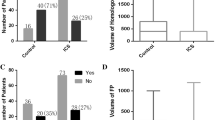
Statistical modelling showed that women who were mandated IOCS had lower rates of post-partum iron infusion (odds ratio (OR) = 0.31, 95% CI = 0.12 to 0.80, P = 0.016) than women in the Usual Care group (Table 3). After weighting, the length of stay was not different between the groups (geometric mean ratio = 0.93, 95% CI = 0.86 to 1.004, p = 0.063). These results were confirmed by the ITS analysis (P = 0.012 and P = 0.23 for post-partum iron and length of stay, respectively). Figure 1 shows average weekly cell salvage and post-partum iron rates and length stay before and after mandated IOCS.
Discussion
The introduction of routine IOCS for caesarean deliveries during the early phase of the COVID-19 pandemic resulted in higher processing rates and reinfusion volumes of autologous RBC. Consequently, post-partum Hb values were improved and anemia less prevalent in women from this cohort. Intravenous iron prescription occurred less often after delivery, and there was no difference in allogeneic RBC transfusion rates between the two groups. These findings add to the existing evidence demonstrating the safety and efficacy of autologous salvaged blood in the context of LSCS, through the conservation of patient blood and maintained iron stores.
After accounting for the difference between patients at baseline, mandating cell salvage reduced post-operative iron infusion rates. Length of stay, however, appeared to be in decline prior to the COVID restrictions and there was no significant difference between the time periods.
Although not contradicted in this study, IOCS has been shown to reduce the need for allogeneic transfusion of RBC in other surgical settings. [6, 15] In 2012, Liumbruno et al. labelled IOCS “the most practical, effective and useful blood conservation technique in obstetrics” [15]. Due to comprehensive staff education and general enthusiasm for the implementation of PBM among staff across disciplines at our hospital, IOCS was introduced in 2013 for elective and emergency surgery. It quickly became an essential tool for managing maternal bleeding, combined with a specific obstetric hemorrhage bleeding protocol and point-of-care coagulation monitoring. This comprehensive obstetric PBM approach resulted in the lowest RBC transfusion rate in South Australia (personal communication, South Australian Blood, Organ and Tissue Programs). The trial period of mandated IOCS in response to the limited blood availability during the COVID-19 pandemic, demonstrated no further reduction in RBC transfusion rate. It was, however, associated with an improvement in Hb status and reduced rates of post-partum iron deficiency anemia in the Mandated IOCS group compared to those receiving Usual Care. These outcomes may explain the 69% four-month trial period highlight the benefits for the patient-derived from the utilization of IOCS for LSCS.
This study has highlighted the valuable contribution of IOCS in obstetric patient care. The mandated use of IOCS enhanced the effectiveness of an established and successful PBM approach, adding to the pre-existing strong body of evidence. Over the last decade, the outcomes for over 2000 cases of autologous RBC re-infusion after red blood cell recovery during LSCS have been published in the literature. This research has demonstrated the safety, efficacy, and benefits of IOCS, [6, 9,10,11, 16, 17] regardless of technique, for example differing suction setups, the use or absence of leucocyte depletion filters, and selective versus routine cell salvage provision.
Maternal mortality is increasing in the United States and many parts of the world [10]. Obstetric hemorrhage is the most common cause of maternal death and consequently peri-partum RBC transfusion rates are on the rise, accounting for approximately 3% of all RBC transfusions [18]. It is important to emphasize that these transfusion events occur in young women, who potentially suffer long-term consequences from exposure to allogeneic RBC’s [19, 20]. Bleeding during childbirth can be life-threatening and is notoriously difficult to predict [21]. IOCS in obstetrics is recommended by organizations such as The Association of Anaesthetists of Great Britain and Ireland, The National Institute for Health and Clinical Excellence, The American College of Obstetrics & Gynaecology, The Australian National Blood Authority, the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis and the German/Austrian/Swiss Societies of Gynecology and Obstetrics (DGGG, OEGGG, SGGG) [22,23,24,25,26,27].
However, the availability of this important tool is far from universal and often absent, even in many large obstetric services. In a recent publication, Hofmann et al. screened international efforts and strategies to identify the four main drivers for successful PBM implementation: patient outcomes, cost savings, preventing blood shortages and patient safety [28]. Whether routine IOCS in obstetrics is cost-saving remains unclear, the use of red cell recovery and autotransfusion clearly supports improved patients outcomes, improved patient safety and prevention of blood shortages, all of which have been urgently required during the COVID-19 pandemic [4, 29].
Withholding the provision of IOCS in obstetrics deprives women of the safest form of RBC transfusion, exposing them to preventable anemia and the well-known risks of allogeneic transfusion. Clinicians from around the world caring for women during childbirth should strengthen their efforts to establish IOCS as an integrated modality on a much broader scale [30,31,32].
Limitations
The two-month trial of mandated IOCS at our site limited the number of patients included in this study. While we did observe higher rates of re-infusion in the mandated group, a much larger sample size would be required to detect any difference in the donor blood transfusion rates. This sample size would depend on the baseline rate of transfusion, which is already low at our site given our established PBM program. Nonetheless, the benefits of IOCS were still apparent, including lower rates of iron-deficient anemia, a higher post-operative Hb.
In the Usual Care group, cell salvage was initiated for 31% (n = 62/203) of women while 86% of women (n = 196/227) had cell salvage initiated following the mandate. The uptake of cell salvage following the introduction of the mandate started at 48% in week 1, increasing to 93% in week 2. It then remained high throughout the two-month period, ranging from 75 to 96%. Reasons for IOCS not being used include some staff being unaware of new trial clinical protocol and clinician preference.
Interestingly in this study, the EBL was lower in the IOCS Mandate group compared to the Usual Care group. Multiple factors may have contributed to this finding, including the higher number of emergency caesarean sections in the Usual Care IOCS group. In addition, the single-cell salvage set-up may have limited observation of any blood loss, which is already notoriously inaccurate as it relied on visual assessment.
Due to a functional PBM program at our hospital, cell salvage was already occurring in the routine care group, before it was mandated. This represents confounding and may reduce the significance of our observed results. However, it was not the purpose of the study to test cell salvage vs no cell salvage.
The authors acknowledge that while statistically significant results were observed, the clinical significance of this is less certain. We also acknowledge that a study with a significantly larger sample size is required to observe differences in donor transfusion rates and indeed overall cost-effectiveness of mandated IOCS.
Conclusions
Routine cell salvage provision during LSCS associated with increased post-operative hemoglobin and reduced anemia incidence which may contribute to an avoidance of limited blood products. We also observed a reduction in post-partum iron infusions. Currently, there are a number of barriers to the routine use of IOCS at some sites, including a lack of awareness and funding to support IOCS establishment and protocols for routine IOCS. The improved clinical outcomes we have observed at our site and presented in this manuscript suggest there may be a benefit of using routine intraoperative cell salvage in LSCS. Future research will be conducted to evaluate the economic outcomes associated with increased cell salvage use.
Data availability
All data associated with the manuscript and not included in tables, figures or supplemental material are available on request to the corresponding author.
References
Cai X, Ren M, Chen F, Li L, Lei H, Wang X (2020) Blood transfusion during the COVID-19 outbreak. Blood Trans Trasfusione del sangue 18(2):79–82. https://doi.org/10.2450/2020.0076-20
Mascaretti L, De Angelis V, Berti P (2020) The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic and Transfusion Medicine: reflections from Italy. Blood Trans Trasfusione del sangue 18(2):77–78. https://doi.org/10.2450/2020.0071-20
Ngo A, Masel D, Cahill C, Blumberg N, Refaai MA (2020) Blood banking and transfusion medicine challenges during the COVID-19 pandemic. Clin Lab Med 40(4):587–601. https://doi.org/10.1016/j.cll.2020.08.013
Shander A, Goobie SM, Warner MA, Aapro M, Bisbe E, Perez-Calatayud AA, Callum J, Cushing MM, Dyer WB, Erhard J, Faraoni D, Farmer S, Fedorova T, Frank SM, Froessler B, Gombotz H, Gross I, Guinn NR, Haas T, Hamdorf J, Isbister JP, Javidroozi M, Ji H, Kim YW, Kor DJ, Kurz J, Lasocki S, Leahy MF, Lee CK, Lee JJ, Louw V, Meier J, Mezzacasa A, Munoz M, Ozawa S, Pavesi M, Shander N, Spahn DR, Spiess BD, Thomson J, Trentino K, Zenger C, Hofmann A, International Foundation of Patient Blood M, Society for the Advancement of Blood Management Work G (2020) Essential role of patient blood management in a pandemic: a call for action. Anesth Analg 131(1):74–85. https://doi.org/10.1213/ANE.0000000000004844
Meybohm P, Froessler B, Goodnough LT, Klein AA, Munoz M, Murphy MF, Richards T, Shander A, Spahn DR, Zacharowski K (2017) “Simplified international recommendations for the implementation of patient blood management” (SIR4PBM). Perioperative Med (London, England) 6(1):5. https://doi.org/10.1186/s13741-017-0061-8
Meybohm P, Choorapoikayil S, Wessels A, Herrmann E, Zacharowski K, Spahn DR (2016) Washed cell salvage in surgical patients: A review and meta-analysis of prospective randomized trials under PRISMA. Medicine 95(31):e4490. https://doi.org/10.1097/MD.0000000000004490
Scott AV, Nagababu E, Johnson DJ, Kebaish KM, Lipsitz JA, Dwyer IM, Zuckerberg GS, Barodka VM, Berkowitz DE, Frank SM (2016) 2,3-Diphosphoglycerate concentrations in autologous salvaged versus stored red blood cells and in surgical patients after transfusion. Anesth Analg 122(3):616–623. https://doi.org/10.1213/ANE.0000000000001071
Frank SM (2011) Who benefits from red blood cell salvage?–Utility and value of intraoperative autologous transfusion. Transfusion 51(10):2058–2060. https://doi.org/10.1111/j.1537-2995.2011.03293.x
Sullivan IJ, Ralph CJ (2019) Obstetric intra-operative cell salvage: a review of an established cell salvage service with 1170 re-infused cases. Anaesthesia 74(8):976–983. https://doi.org/10.1111/anae.14630
Waters JH, Beck S, Yazer MH (2019) How do I perform cell salvage in obstetrics? Transfusion 59(7):2199–2202. https://doi.org/10.1111/trf.15352
Liu Y, Li X, Che X, Zhao G, Xu M (2020) Intraoperative cell salvage for obstetrics: a prospective randomized controlled clinical trial. BMC Pregnancy Childbirth. https://doi.org/10.1186/s12884-020-03138-w
National Blood Authority COVID19 blood management poster, 2020. Available at https://www.blood.gov.au/download-covid19-blood-management-poster. Accessed 21/10/2021.
World Health Organization. The global prevalence of anaemia in 2011. Geneva: WHO, 2015. Available at https://www.who.int/publications/i/item/9789241564960, Accessed 23/10/2021.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335(7624):806–808. https://doi.org/10.1136/bmj.39335.541782.AD
Liumbruno GM, Liumbruno C, Rafanelli D (2012) Autologous blood in obstetrics: where are we going now? Blood Trans rasfusione del sangue 10(2):125–147. https://doi.org/10.2450/2011.0010-11
Wang R, Luo T, Liu Z, Fan J, Zhou G, Wu A, Liu J (2020) Intraoperative cell salvage is associated with reduced allogeneic blood requirements and has no significant impairment on coagulation function in patients undergoing cesarean delivery: a retrospective study. Arch Gynecol Obstet 301(5):1173–1180. https://doi.org/10.1007/s00404-020-05500-x
Khan KS, Moore PAS, Wilson MJ, Hooper R, Allard S, Wrench I, Beresford L, Roberts TE, McLoughlin C, Geoghegan J, Daniels JP, Catling S, Clark VA, Ayuk P, Robson S, Gao-Smith F, Hogg M, Lanz D, Dodds J, group Ss (2017) Cell salvage and donor blood transfusion during cesarean section: A pragmatic, multicentre randomised controlled trial (SALVO). PLoS Med 14(12):e1002471. https://doi.org/10.1371/journal.pmed.1002471
Patterson JA, Roberts CL, Bowen JR, Irving DO, Isbister JP, Morris JM, Ford JB (2014) Blood transfusion during pregnancy, birth, and the postnatal period. Obstet Gynecol 123(1):126–133. https://doi.org/10.1097/AOG.0000000000000054
Thurn L, Wikman A, Westgren M, Lindqvist PG (2019) Incidence and risk factors of transfusion reactions in postpartum blood transfusions. Blood Adv 3(15):2298–2306. https://doi.org/10.1182/bloodadvances.2019000074
Ukah UV, Platt RW, Potter BJ, Paradis G, Dayan N, He S, Auger N (2020) Obstetric haemorrhage and risk of cardiovascular disease after three decades: a population-based cohort study. BJOG 127(12):1489–1497. https://doi.org/10.1111/1471-0528.16321
Collis R, Guasch E (2017) Managing major obstetric haemorrhage: Pharmacotherapy and transfusion. Best Pract Res Clin Anaesthesiol 31(1):107–124. https://doi.org/10.1016/j.bpa.2017.02.001
Munoz M, Stensballe J, Ducloy-Bouthors AS, Bonnet MP, De Robertis E, Fornet I, Goffinet F, Hofer S, Holzgreve W, Manrique S, Nizard J, Christory F, Samama CM, Hardy JF (2019) Patient blood management in obstetrics: prevention and treatment of postpartum haemorr hage A NATA consensus statement. Blood Trans Trasfusione del sangue 17(2):112–136. https://doi.org/10.2450/2019.0245-18
Klein AA, Bailey CR, Charlton AJ, Evans E, Guckian-Fisher M, McCrossan R, Nimmo AF, Payne S, Shreeve K, Smith J, Torella F (2018) Association of Anaesthetists guidelines: cell salvage for peri-operative blood conservation 2018. Anaesthesia 73(9):1141–1150. https://doi.org/10.1111/anae.14331
Authority NB (2015) Patient Blood Management Guidelines: Module 5 Obstetrics and Maternity. https://www.blood.gov.au/pbm-module-5. Accessed 5/10/2021 2021
Committee on Obstetric P (2002) ACOG committee opinion. Placenta accreta. Number 266, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 77 (1) 77–78. doi:https://doi.org/10.1016/s0020-7292(02)80003-0
(NICE) TNIfHaCE (2005) Intraoperative blood cell salvage in obstetrics. https://www.nice.org.uk/guidance/IPG144. Accessed 6/10/2021 2021
Schlembach D, Helmer H, Henrich W, von Heymann C, Kainer F, Korte W, Kuhnert M, Lier H, Maul H, Rath W, Steppat S, Surbek D, Wacker J (2018) Peripartum Haemorrhage, Diagnosis and Therapy. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry No. 015/063, March 2016). Geburtshilfe Frauenheilkd 78 (4):382–399. doi:https://doi.org/10.1055/a-0582-0122
Hofmann A, Spahn DR, Holtorf AP, (2021) Making patient blood management the new norm (al) as experienced by implementors in diverse countries. BMC Health Serv Res 21 (1): 634. https://doi.org/10.1186/s12913-021-06484-3
Gehrie EA, Frank SM, Goobie SM (2020) Balancing supply and demand for blood during the COVID-19 pandemic. Anesthesiology 133(1):16–18. https://doi.org/10.1097/ALN.0000000000003341
VanderMeulen H, Strauss R, Lin Y, McLeod A, Barrett J, Sholzberg M, Callum J (2020) The contribution of iron deficiency to the risk of peripartum transfusion: a retrospective case control study. BMC Pregnancy Childbirth 20(1):196. https://doi.org/10.1186/s12884-020-02886-z
Teichman J, Nisenbaum R, Lausman A, Sholzberg M (2021) Suboptimal iron deficiency screening in pregnancy and the impact of socioeconomic status in a high-resource setting. Blood Adv 5(22):4666–4673. https://doi.org/10.1182/bloodadvances.2021004352
Butwick AJ, McDonnell N (2021) Antepartum and postpartum anemia: a narrative review. Int J Obstet Anesth. https://doi.org/10.1016/j.ijoa.2021.102985
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by TF, ET, AL, RS, NH, TLK and BF. The first draft of the manuscript was written by TF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
BF reports speaker fees from Vifor Pharma, Pfizer and the Heart Team Education Association outside the submitted work. TF, ET, AG, AP, RS, TK, NH have nothing to disclose.
Ethical approval
This study was approved by our Institutional Review Board (IRB), (CALHN Reference Number: 14994). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was waived by the IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fox, T.P., Timpani, E., Green, A. et al. Association between routine cell salvage use for lower segment caesarean section and post-operative iron infusion and anemia. Arch Gynecol Obstet 309, 1935–1941 (2024). https://doi.org/10.1007/s00404-023-07082-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07082-w