Abstract
Purpose
Vulvar lichen sclerosus (LS) is a chronic debilitating inflammatory skin disease. Today, the gold standard is a life-long topical steroid treatment. Alternative options are highly desired. We present a study protocol of a prospective, randomized, active-controlled, investigator-initiated clinical trial comparing a novel non-invasive dual Nd:YAG/Er:YAG laser therapy with the gold standard for the management of LS.
Methods
We recruited 66 patients, 44 in the laser arm and 22 in the steroid arm. Patients with a physician-administered clinical LS score ≥ 4 were included. Participants received either four laser treatments 1–2 months apart, or 6 months of topical steroid application. Follow-ups were planned at 6, 12, and 24 months. The primary outcome looks at the efficacy of the laser treatment at the 6-month follow-up. Secondary outcomes look at comparisons between baseline and follow-ups within the laser or the steroid arm, and comparisons between laser vs. steroid arm. Objective (LS score, histopathology, photo documentation) and subjective (Vulvovaginal Symptoms Questionnaire, symptom VAS score, patient satisfaction) measurements, tolerability, and adverse events are evaluated.
Conclusion
The findings of this trial have the potential to offer a novel treatment option for LS. The standardized Nd:YAG/Er:YAG laser settings and the treatment regime are presented in this paper.
Clinical trial identification number
NCT03926299.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Presentation of a novel non-invasive double laser therapy to treat vulvar lichen sclerosus. In a randomized controlled trial with a follow-up of two years, the new laser therapy is evaluated as an alternative to the gold standard treatment with topical steroid. |
Introduction
Lichen sclerosus (LS) is a chronic inflammatory skin disease most commonly found in adult women. It is almost exclusively restricted to the anogenital region [1]. The incidence rate is highest in postmenopausal women (24–53 of 100,000 per year) and the prevalence is 2–7% [2]. The typical symptom is vulvar itching, further symptoms include vulvar burning or stinging, vulvar pain, and dyspareunia [2], with a high impact on quality of life. A significant percentage of cases can also be asymptomatic (15–40%) [3], and the diagnosis may be delayed. LS is a progressive autoimmune disease with an intense inflammation process leading to destruction of the loose connective tissue and to clear macroscopic changes of the vulvar anatomy with white atrophic plaques, depigmentation, erosions, hyperkeratosis, fissures, agglutination with labial resorption, clitoral phimosis and introital stenosis [4]. When untreated, the lifetime risk for a vulvar carcinoma is 5% [5].
The application of topical steroids is the gold standard for treating female genital LS [6]. There is no standardized treatment regime; however, the potent steroid clobetasol propionate, 0.05% ointment or cream once or twice daily for the first few months with a reduction of application frequency for the subsequent months is recommended to avoid skin thinning. A maintenance treatment prevents severe relapses [6] and tumor progression [5]. Treatment improves symptoms and can reverse early signs. However, severe architectural changes are irreversible [6]. Additional moisturizers improve symptoms.
Side effects of steroid treatment include skin atrophy, superimposed infections and adrenal insufficiency, and furthermore, a strict compliance with the treatment regime is required [7]. Advanced stages of LS with an epithelial hyperkeratosis and a band-shaped subepithelial sclerosis can be refractory to standard steroid treatment, most likely because the topically applied medication cannot penetrate the broad tissue layers [8, 9]. There is a great interest for alternative therapies among patients and practitioners.
Laser therapy of the vulva might be such an alternative, potentially even circumventing some of the disadvantages of steroid therapy. A recent review on laser treatment for LS identified three randomized controlled trials (RCTs), three cohort studies, and four case reports [10]. In nine studies including two RCTs [7, 11], the fractional ablative CO2 laser was used, one RCT used the non-ablative Nd:YAG laser [12] and one case report the fractional ablative Er:YAG laser [8]. Laser therapy improves symptoms in most studies [13]. However, methodology and results were too heterogeneous, patients too few and follow-up periods too short for a recommendation [13].
Fractional ablative CO2 laser therapy (10′600 nm) sets visible, possibly painful micro-wounds by superficial skin evaporation [7] and makes thin channels into the tissue to facilitate temperature penetration. Heat stimulates connective tissue remodeling, collagen production, and re-vascularization [14]. In contrast, the non-ablative Nd:YAG laser (1064 nm) has very low absorption in water and thus, penetrates deeply into tissue. Long Nd:YAG laser pulses do not damage the skin surface, but generate a slow gentle temperature increase in deeper lying structures (> 5 mm) and induce the heat response [12, 15, 16]. Two RCTs demonstrated significant between-group and in-group improvement favoring the CO2 or Nd:YAG lasers over the steroid treatment [11, 12]. The third RCT compared CO2 laser vs. sham treatment, and found no significant between-group difference in histopathologic change [7]. Long-term and high-quality RCTs are needed before laser can be considered a routine treatment for LS [10, 13].
The objective of this study was to assess efficacy, safety, and sustainability of a novel dual Nd:YAG/Er:YAG laser treatment concept for LS. The heat generated by the Nd:YAG laser is expected to penetrate through hyperkeratosis, epithelium, and subepithelial sclerosis, reaching the deeper regenerative tissue area to reduce inflammation and induce collagen remodeling and neovascularization. Additionally, the ablative Er:YAG laser is expected to reduce superficial hyperkeratosis and other skin irregularities. The study was designed as a prospective, single center, investigator-initiated RCT, using topical steroid, the current gold standard, as the comparator. The study hypothesis is that the Nd:YAG/Er:YAG laser combination is effective and can achieve similar results as the standard steroid treatment.
Methods
Design
This study is a post-market, single center, prospective, randomized, active-controlled investigator-initiated clinical trial. It was performed at an ambulatory tertiary referral center for bladder, vulvar, and pelvic floor disorders at the Department of Gynecology and Obstetrics, Cantonal Hospital Frauenfeld, Switzerland. All women with clinical presentation of LS were asked to participate. The efficacy and safety of a novel dual Nd:YAG/Er:YAG laser (FotonaSmooth SP® Spectro laser (Model M021-4AF/3)) were compared to the gold standard therapy with topical steroids to treat vulvar LS. The study followed the Declaration of Helsinki, received ethical approval (EKOS 19/056, BASEC-ID: 2019–00,634), and was registered at clinicaltrials.gov (NCT03926299).
Inclusion criteria at time of randomization
-
(1)
Woman age 18 or older.
-
(2)
Clinical diagnosis for LS (chronic pruritus of non-fungal origin, burning sensations, soreness, and dyspareunia, no oral lesions).
- (3)
-
(4)
Agreement for two vulvar punch biopsies before and after treatment.
Exclusion criteria at time of randomization
-
(1)
Concomitant steroid, calcineurin inhibitor or any other topical or systemic treatment for LS (therapy has to be stopped ≥ 2 weeks before the screening visit).
-
(2)
< 3 months since start of vaginal estrogen treatment.
-
(3)
Malignant disease as the cause of the symptoms, including precursors, e.g., differentiated exophytic vulvar intraepithelial lesion or vulvar acanthosis with altered differentiation.
-
(4)
BMI > 35 kg/m2.
-
(5)
Acute infection (fungal, bacterial, viral) of the vulva, vagina or bladder.
-
(6)
Presence of contraindications for the laser treatment or topical steroid treatment (e.g., using drugs causing photosensitivity or a hypersensitivity/allergy to clobetasol propionate)
-
(7)
≤ 3 months since labor, miscarriage or an operation in the lower abdomen.
-
(8)
Pregnancy, breast feeding or the intention to become pregnant during the study.
-
(9)
Lack of safe contraception for the study duration.
-
(10)
Participation in another study with investigational drug within the 30 days preceding or during the present study.
-
(11)
Enrollment of the investigator, his/her family members, employees and other dependent persons.
-
(12)
Unwillingness or inability to comply with study plan.
-
(13)
Unwillingness or inability to consent.
Withdrawal management
Participants with one of the following criteria were allowed or required to withdraw from the study:
-
(1)
Voluntary withdrawal at any time without giving any reason.
-
(2)
Withdrawal by the investigator if it was in the best interest for the patient, e.g., after an adverse event such as the diagnosis of a disease, or when her safety was at risk.
-
(3)
Non-compliance to the study protocol.
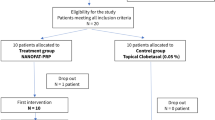
If a patient had withdrawn before completion of the laser or the steroid therapy, she was replaced.
Protocol
Study plan
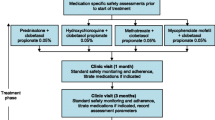
At the screening visit, eligibility was evaluated, patients gave informed consent. Randomization and allocation were done at baseline. The laser intervention group received four laser treatments, the comparator group received topical steroids for 6 months. Follow-up visits were at 6, 12, and 24 months after baseline. Vulvar biopsies were taken at the screening visit and at the 6-month follow-up (Table 1). Steroid therapy had to be stopped ≥ 2 weeks before the first biopsy.
Description of the intervention
Laser arm
The Nd:YAG/Er:YAG laser therapy was applied four times in an ambulatory setting: at baseline and after 1, 2, and 4 months (Table 1). The laser settings are shown in Table 2. One laser session takes 10–15 min. Topical lidocaine cream is optional and can be applied when needed.
Steroid arm
Topical steroid (clobetasol 0.05%) cream was applied as the active control treatment for 6 months. Patients started with a high dose which subsequently was reduced after 2 and 4 months (Table 1), following this treatment regime*:
-
Phase 1 (first and second month): at four evenings of the week, every week.
-
Phase 2 (third and fourth month): at four evenings of the week, every other week.
-
Phase 3 (fifth and sixth month): at four evenings of the week, first week of the month.
*modified from the University Hospital Zurich scheme [18].
The treatment was complemented with a moisturizing cream twice a day (morning and evening), except on the evenings when steroid cream was applied. Treatment success depends on the patients’ compliance.
Primary outcome
The primary outcome of this study was to assess the efficacy of the Nd:YAG/Er:YAG laser combination therapy to treat LS by evaluating the change of the LS score from baseline to the 6-month follow-up. The LS score is an objective, validated, physician-administered clinical score to measure the severity of vulvar LS [4, 7, 19]. A physician assesses each of the six criteria (1) erosions, (2) hyperkeratosis, (3) fissures, (4) agglutination, (5) stenosis, and (6) atrophy on a scale from 0 (normal/none), 1 (a few signs/moderate) to 2 (clear signs/severe) [17]. Hence, the LS score ranges from 0 to 12. An LS score ≥ 4 identifies LS with a probability of > 90% [4]. Individual characteristics for “none”, “moderate”, or “severe” are defined [4].
Secondary outcomes
Secondary outcomes were assessed as comparisons between baseline and follow-ups within the laser or the steroid group, and as comparisons between laser vs. steroid group. The following measurements were evaluated:
-
Demographics and medical history.
-
Clinical examination, photo documentation, and clinical sketch of the vulva to localize symptoms, biopsy, and laser treatment.
-
Subjective Vulvovaginal Symptoms Questionnaire (VSQ) [20], a validated evaluation of the four domains “symptoms” (seven questions), “emotions” (four questions), “life impact” (four questions), and “sexual impact” (five questions, four of them for sexually active women). The score ranges from 0 (no symptoms) to 16 or 20 for sexually active women (severe symptoms).
-
Subjective evaluation of symptom strength (VAS 0-10) of the four typical symptoms: vulvar itching, vulvar burning or stinging, vulvar pain and dyspareunia.
-
Patient satisfaction by the Patient Global Impression of Improvement (PGI-I) questionnaire [21].
-
Discomfort/pain (VAS 0–10) and adverse events during and immediately after each laser session.
-
Total joule counts and number of pulses per laser session.
-
Device deficiencies.
-
Treatment history between visits.
-
Adverse events. Patients are asked systematically at each visit. Documentation includes dates of event, treatment, resolution, assessment of seriousness and causal relationship to device and/or study procedure.
-
Histopathological evaluation of vulvar biopsies by an external, independent expert pathologist.
Numbers
Sample size calculation was performed for the primary endpoint, the clinical LS score at 6 months. A total of 34 patients in the laser arm would provide 80% power to detect a medium effect size of 0.5 [22] with a two-sided alpha set at 5%. Assuming a drop-out rate of 10–15%, 40 patients were needed in the laser arm.
For a superiority/inferiority test between groups, a total sample size of 60 patients—40 in the laser group, 20 in the steroid group—would provide 80% power to detect a large effect size of 0.8 [22] with a two-sided alpha set at 5%. Adding again a buffer of 10% for drop-outs, we aimed to recruit 66 patients, 44 in the laser group and 22 in the steroid group.
Randomization
At baseline, the study participants were randomly allocated to the laser or the steroid arm in a 2:1 ratio. Stratification was done according to the severity of the clinical LS score: clinical LS score 4, 5 or 6 (LSL low) vs. LS score > 6 (LSH high). Block randomization was applied for each stratum. An independent scientist without patient contact prepared the randomization process. Study doctors got sealed, consecutively labeled envelopes for each stratum. Envelopes were opened in subsequent order at baseline before the first treatment. The allocation to the treatment arm was noted on the case report form at baseline. Blinding of patients for the allocated intervention was not possible. Two persons independently scored the clinical findings. The investigating pathologist was blinded for allocation.
Statistical methods
Baseline comparison of the two treatment arms is assessed by one-way ANOVA or t test (for continuous variables), Poisson regression (for count data) or Fisher test (for categorical or binomial data). If the assumption of normal distribution is violated, non-parametric alternatives (Friedman, Kruskal–Wallis, and Wilcoxon Signed-Rank tests) can be used for the analysis. For all tests, a significance level of alpha = 5% is applied.
The analysis of changes to baseline is done with the t test, testing the null hypothesis that there is no difference between baseline and follow-ups. Further analysis is done testing the null hypothesis that there are no differences between the two treatment arms (t test).
For subgroup analysis, mean differences between baseline and follow-ups are stratified according to the subgroup findings at baseline. The interaction effect of baseline characteristics on treatment effect is investigated by linear regression.
Follow-ups
Follow-up visits were at 6 and 12 months after baseline, and a telephone interview was planned at 24 months. At 6 months, the primary outcome was evaluated and a second vulvar biopsy was taken (Table 1). Between 6 and 12 months, patients in the steroid arm were offered four optional laser treatments, patients in the laser arm could optionally request further laser treatments, and patients in both arms were allowed to optionally use topical steroids. At 12 months, all study parameters including the treatment history between 6 and 12 months were registered. At the telephone interview at 24 months, subjective parameters and the treatment history between 12 and 24 months were recorded.
Discussion
Today, laser therapy is propagated as a solution for a variety of indications, including gynecological diseases. Everybody uses the laser. However, based on the actual data situation, experts cannot give recommendations yet, but request more studies, ideally RCTs with longer observation periods [10, 23, 24].
The presented study was designed as an RCT with a long follow-up period to evaluate a novel laser treatment for LS. In a standardized research setting, the study will give answers regarding tolerability and safety of a new non-invasive double laser concept for the treatment of vulvar LS, efficacy and durability of the laser treatment with an observation time of 2 years, patient satisfaction and therapy adherence, effect of the laser treatment on the physician-evaluated clinical outcome in comparison to subjective symptom changes and improvement of quality of life, efficacy of the laser treatment in comparison to the current gold standard with topical steroid treatment, identification of patient groups best suited for laser treatment, and histopathological changes after laser and after steroid therapy.
The study design aimed to minimize the drop-out ratio. It may help that the patients in both study arms receive an active therapy rather than a sham/placebo treatment. Suffering should also be minimized by allowing the use of any symptom relieve after the 6-month visit. Frequent study visits for both study arms further help to keep patients involved, and the prospect of laser treatment after 6 months encourage patients in the steroid arm to comply with the study setting.
The relatively low patient number at just one trial center can be interpreted as a trial limitation. However, the suggested treatment is new and must be tested before upscaling. Furthermore, this study setting allows a uniform patient inclusion, treatment, and data acquisition and is expected to deliver consistent data.
The results of this study are highly expected from numerous practitioners and—above all—from the many patients with a long disease history. The new double Nd:YAG/Er:YAG laser treatment is minimally invasive, free of steroids, and requires only four interventions. Furthermore, the RCT setting will show whether the dual laser combination can be an alternative to the standard steroid treatment.
Change history
24 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00404-023-07162-x
References
Powell JJ, Wojnarowska F (1999) Lichen sclerosus. Lancet 353:1777–1783. https://doi.org/10.1016/s0140-6736(98)08228-2
Halonen P, Jakobsson M, Heikinheimo O, Gissler M, Pukkala E (2020) Incidence of lichen sclerosus and subsequent causes of death: a nationwide finnish register study. BJOG 127:814–819. https://doi.org/10.1111/1471-0528.16175
Perez-Lopez FR, Vieira-Baptista P (2017) Lichen sclerosus in women: a review. Climacteric 20:339–347. https://doi.org/10.1080/13697137.2017.1343295
Günthert AR, Duclos K, Jahns BG, Krause E, Amann E, Limacher A, Mueller MD, Juni P (2012) Clinical scoring system for vulvar lichen sclerosus. J Sex Med 9:2342–2350. https://doi.org/10.1111/j.1743-6109.2012.02814.x
Lee A, Bradford J, Fischer G (2015) Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol 151:1061–1067. https://doi.org/10.1001/jamadermatol.2015.0643
Kirtschig G, Cooper S, Aberer W, Gunthert A, Becker K, Jasaitiene D, Chi CC, Kreuter A, Rall K, Riechardt S, Casabona F, Powell J, Brackenbury F, Erdmann R, Lazzeri M, Barbagli G, Wojnarowska F (2017) Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol 31:e81–e83. https://doi.org/10.1111/jdv.13740
Mitchell L, Goldstein AT, Heller D, Mautz T, Thorne C, Joyce Kong SY, Sophocles ME, Tolson H, Krapf JM (2021) Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol 137:979–987. https://doi.org/10.1097/AOG.0000000000004409
Hobson JG, Ibrahim SF, Mercurio MG (2019) Recalcitrant vulvar lichen sclerosus treated with erbium YAG laser. JAMA Dermatol 155:254–256. https://doi.org/10.1001/jamadermatol.2018.4461
Gadaldi K, Cazzaniga S, Feldmeyer L, Krause E, Günthert AR, Beltraminelli H (2020) Genital lichen sclerosus in women: a histopathological analysis of 38 criteria. J Eur Acad Dermatol Venereol 34:e418–e420. https://doi.org/10.1111/jdv.16368
Mortensen OE, Christensen SE, Lokkegaard E (2022) The evidence behind the use of LASER for genitourinary syndrome of menopause, vulvovaginal atrophy, urinary incontinence and lichen sclerosus: a state-of-the-art review. Acta Obstet Gynecol Scand 101:657–692. https://doi.org/10.1111/aogs.14353
Burkett LS, Siddique M, Zeymo A, Brunn EA, Gutman RE, Park AJ, Iglesia CB (2021) Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol 137:968–978. https://doi.org/10.1097/AOG.0000000000004332
Bizjak Ogrinc U, Sencar S, Luzar B, Lukanovic A (2019) Efficacy of non-ablative laser therapy for lichen sclerosus: a randomized controlled trial. J Obstet Gynaecol Can 41:1717–1725. https://doi.org/10.1016/j.jogc.2019.01.023
Tasker F, Kirby L, Grindlay DJC, Lewis F, Simpson RC (2021) Laser therapy for genital lichen sclerosus: a systematic review of the current evidence base. Skin Health Dis 1:e52. https://doi.org/10.1002/ski2.52
Pagano T, Conforti A, Buonfantino C, Schettini F, Vallone R, Gallo A, Avino L, Alviggi C, De Placido G, Sopracordevole F (2020) Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause 27:418–422. https://doi.org/10.1097/GME.0000000000001482
Lukac M, Vizintin Z, Pirnat S, Nemes K (2011) New skin treatment possibilities with PIANO mode on an Nd:YAG laser. J Las Health Acad 1:22–32
Vizintin Z (2022) Basic principles and physics of Gyno4D. In: Gambacciani M, Gaspar A, Gaviria J (eds) The laser essentials - the cookbook for functional gynecology and aesthetics. Edizione Minerva Medica, pp 1–8
Günthert AR (2020) Clinical scoring system for vulvar lichen sclerosus. Erratum J Sex Med 17:1825. https://doi.org/10.1016/j.jsxm.2020.06.021
Dedes I, Ghisu G-P, Fink D (2019) Lichenoide Vulvaerkrankungen. Lichen sclerosus und Lichen planus: differenzialdiagnostik und behandlung. Gynäkologie 1:11–16
Naswa S, Marfatia YS (2015) Physician-administered clinical score of vulvar lichen sclerosus: a study of 36 cases. Indian J Sex Transm Dis AIDS 36:174–177. https://doi.org/10.4103/0253-7184.167169
Erekson EA, Yip SO, Wedderburn TS, Martin DK, Li FY, Choi JN, Kenton KS, Fried TR (2013) The vulvovaginal symptoms questionnaire: a questionnaire for measuring vulvovaginal symptoms in postmenopausal women. Menopause 20:973–979. https://doi.org/10.1097/GME.0b013e318282600b
Srikrishna S, Robinson D, Cardozo L (2010) Validation of the patient global impression of improvement (PGI-I) for urogenital prolapse. Int Urogynecol J 21:523–528. https://doi.org/10.1007/s00192-009-1069-5
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Publishers, Hillsdale
Ruffolo AF, Braga A, Torella M, Frigerio M, Cimmino C, De Rosa A, Sorice P, Castronovo F, Salvatore S, Serati M (2022) Vaginal laser therapy for female stress urinary incontinence: New solutions for a well-known issue - a concise review. Medicina (Kaunas) 58:512. https://doi.org/10.3390/medicina58040512
Phillips C, Hillard T, Salvatore S, Toozs-Hobson P, Cardozo L (2020) Lasers in gynaecology. Eur J Obstet Gynecol Reprod Biol 251:146–155. https://doi.org/10.1016/j.ejogrb.2020.03.034
Funding
Fotona company lends the laser and provides the laser equipment without any restriction on the study design of this investigator-initiated trial. The authors declare that they received no funds, grants or other support during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Claudia Walser contributed to the ethical application and the statistical design. Marianne Gamper and Volker Viereck wrote the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study received ethical approval (EKOS 19/056, BASEC-ID: 2019-00634), and is registered at clinicaltrials.gov (NCT03926299).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: To correct the error in Table 1 values.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viereck, V., Gamper, M., Regauer, S. et al. Nd:YAG/Er:YAG dual laser vs. topical steroid to treat vulvar lichen sclerosus: study protocol of a randomized controlled trial. Arch Gynecol Obstet 308, 643–649 (2023). https://doi.org/10.1007/s00404-023-07055-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07055-z