Abstract
Purpose
In this study we examined the effects of long-term adaptation to hypoxia on embryonic developmental potential of oocytes collected from women who underwent IVF/ICSI procedures.
Methods
We selected young infertile women who lived in a low-altitude normoxic environment (n = 80, altitude < 500 m) or high-altitude hypoxic environment (n = 100, altitude > 2500 m) for a lengthy period of time and who planned to undergo IVF/ICSI procedures. We then determined the baseline reproductive hormone levels, gonadotropin (Gn) dose and Gn treatment duration during controlled ovarian hyperstimulation (COH), number of oocytes retrieved, number of mature oocytes, oocyte maturation rate, fertilization rate, normal fertilization rate, day (D3) embryo-formation rate, blastocyst formation rate, good-quality formation rate, D5 blastocyst formation rate, and D6 blastocyst formation rate between the two groups.
Results
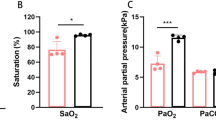
Compared with the low-altitude normoxic group, the various reproductive hormone markers of women in the high-altitude hypoxia group were lower, with LH and T levels significantly reduced (P < 0.05) at 72.29 and 72.44% of the normoxic group, respectively (normoxic group vs. hypoxic group, 5.24 ± 1.61 vs. 3.79 ± 1.21; 0.61 ± 0.18 vs. 0.42 ± 0.15; P < 0.05). During ovarian hyperstimulation, a greater Gn dose and longer Gn treatment duration were required for the hypoxic group to complete COH (normoxic group vs. hypoxic group, 2152.08 IU ± 52.76 vs. 2622.09 IU ± 123.28; 9.96 days ± 1.27 vs. 11.54 days ± 1.34, respectively; P < 0.05). The fertilization, cleavage, and D3 embryo-formation rates tended to be higher in the normoxic group than in the hypoxic group (P > 0.05); while the normal fertilization rate tended to lower than in the hypoxic group (P > 0.05). When we conducted an analysis of blastocyst formation rates at different timepoints, we ascertained that the blastocyst formation rate, usable blastocyst rate, and good-quality blastocyst rate of the hypoxic group were all lower than in the normoxic group, with the difference in usable blastocyst rate the most highly significant (normoxic group vs. hypoxic group, 75.31 ± 5.53 vs. 56.04 ± 6.10%, respectively; P < 0.05). In addition, the D5 and D6 blastocyst-formation rates in the normoxic group were slightly higher than in the hypoxic group, revealing that not only were fewer blastocysts formed in the hypoxic group but that there was also a delay in blastocyst formation.
Conclusion
In young women undergoing IVF/ICSI treatment, long-term hypoxic adaptation required augmented Gn dose and Gn treatment duration during COH, and blastocyst developmental potential was also attenuated.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, Cao Y, Chen X, Zhu Y, Xu S, Chen ZJ, Mol BW, Qiao J (2018) Epidemiology of infertility in China: a population-based study. BJOG 125:432–441
Doody KJ (2021) Infertility treatment now and in the future. Obstet Gynecol Clin North Am 48:801–812
Keefe D, Kumar M, Kalmbach K (2015) Oocyte competency is the key to embryo potential. Fertil Steril 103:317–322
Eichenlaub-Ritter U, Chandley AC, Gosden RG (1988) The CBA mouse as a model for age-related aneuploidy in man: studies of oocyte maturation, spindle formation and chromosome alignment during meiosis. Chromosoma 96:220–226
Wai T, Teoli D, Shoubridge EA (2008) The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 40:1484–1488
Keefe DL, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, Agarwal S, Blasco MA (2005) Telomere length predicts embryo fragmentation after in vitro fertilization in women–toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol 192:1256–60 (discussion 1260-1261)
Moore LG (2021) HYPOXIA AND REPRODUCTIVE HEALTH: reproductive challenges at high altitude: fertility, pregnancy and neonatal well-being. Reproduction 161:F81–F90
von Wolff M, Nakas CT, Tobler M, Merz TM, Hilty MP, Veldhuis JD, Huber AR, Pichler Hefti J (2018) Adrenal, thyroid and gonadal axes are affected at high altitude. Endocr Connect 7:1081–1089
Zheng S, Liu Y, Li P, Tian H (2019) Short-term high-altitude exposure (3600 m) alters the type distribution of sperm deformity. High Alt Med Biol 20:198–202
Moore LG, Young D, McCullough RE, Droma T, Zamudio S (2001) Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol 13:635–644
Gonzales GF, Tapia V, Carrillo CE (2008) Stillbirth rates in Peruvian populations at high altitude. Int J Gynaecol Obstet 100:221–227
Miller S, Tudor C, Thorsten V, Wright L, Varner M (2008) Comparison of maternal and newborn outcomes of Tibetan and Han Chinese delivering in Lhasa. Tibet J Obstet Gynaecol Res 34:986–993
Frisancho AR (1970) Developmental responses to high altitude hypoxia. Am J Phys Anthropol 32:401–407
Crognier E, Villena M, Vargas E (2002) Reproduction in high altitude Aymara: physiological stress and fertility planning? J Biosoc Sci 34:463–473
Vitzthum VJ (2013) Fifty fertile years: anthropologists’ studies of reproduction in high altitude natives. Am J Hum Biol 25:179–189
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 97:9082–9087
Mole DR, Ratcliffe PJ (2008) Cellular oxygen sensing in health and disease. Pediatr Nephrol 23:681–694
Parraguez VH, Urquieta B, Pérez L, Castellaro G, De los Reyes M, Torres-Rovira L, Aguado-Martínez A, Astiz S, González-Bulnes A (2013) Fertility in a high-altitude environment is compromised by luteal dysfunction: the relative roles of hypoxia and oxidative stress. Reprod Biol Endocrinol 11:24
Brunelle JK, Chandel NS (2002) Oxygen deprivation induced cell death: an update. Apoptosis 7:475–482
Braga DP, Setti AS, de Cássia SFR, Machado RB, Iaconelli A Jr, Borges E Jr (2012) Patient selection criteria for blastocyst transfers in extended embryo culture programs. J Assist Reprod Genet 29:1357–1362
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73:1155–1158
Leite RF, Annes K, Ispada J, de Lima CB, Dos Santos ÉC, Fontes PK, Gouveia Nogueira MF, Milazzotto MP (2018) Corrigendum to “oxidative stress alters the profile of transcription factors related to early development on in vitro produced embryos.” Oxid Med Cell Longev 2018:6730857
Takahashi M (2012) Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev 58:1–9
Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, Thornton J, Agarwal A (2004) Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril 82:593–600
Bedaiwy MA, Mahfouz RZ, Goldberg JM, Sharma R, Falcone T, Abdel Hafez MF, Agarwal A (2010) Relationship of reactive oxygen species levels in day 3 culture media to the outcome of in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril 94:2037–2042
Haas J, Bassil R, Samara N, Zilberberg E, Mehta C, Orvieto R, Casper RF (2020) GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod 35:1648–1654
Almog B, Eldar I, Barkan G, Amit A, Wagman I, Levin I (2014) Embryo quality in controlled ovarian stimulation for in vitro fertilization in young poor responders. Gynecol Endocrinol 30:657–659
Funding
This work was supported by Qinghai Plateau Famous Medical Talents Project in 2020 and Western Light Project of Chinese Academy of Sciences in 2020.
Author information
Authors and Affiliations
Contributions
ZFX and XLL conceived the study and designed the experiments. XLL and QDW performed the study and contributed to data collection. BJ and JRJ performed the data analysis and interpreted the results. ZFX wrote the manuscript. ZFX and XLL contributed to the critical revision of article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Ethics Committee of Qinghai Provincial People's Hospital.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, Z., Liu, X., Wang, Q. et al. Effects of high-altitude hypoxia on embryonic developmental potential in women undergoing IVF/ICSI procedures. Arch Gynecol Obstet 307, 1983–1989 (2023). https://doi.org/10.1007/s00404-023-07014-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07014-8