Abstract
Key message
Menstruation of adolescent girls might be influenced by Covid-19 mRNA vaccine, however, the ovarian reserve estimated by AMH is not compromised.
Background
Recent studies have suggested that the acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine causes menstrual abnormalities which led to concerns regarding its influence on the reproductive system. This study aims to investigate the influence of the SARS-CoV-2 mRNA vaccine on gynecologic well-being and future fertility of adolescent girls.
Methods
This is a prospective cohort study conducted at a university affiliated medical center between June and July 2021. Adolescent girls aged 12–16 years who were vaccinated by two Pfizer-BioNTech Covid-19 vaccines (21 days apart) were included in the study. All participants completed a computerized questionnaire regarding their general medical and gynecological background at recruitment and 3 months later. Blood samples were collected for AMH levels before and 3 months following the first mRNA vaccine
Results
The study group consisted of 35 girls, and of them, follow-up was completed by questionnaire and AMH sampling in 35 (90%) and 22 (56%) girls, respectively. Among the 22/35 girls who reported regular menstruation before vaccination, seven (31.8%) experienced irregularities post-vaccination. Four of the eight pre-menarche girls included in the study reported on menarche on follow-up. Median AMH levels were 3.09 (IQR 1.96–4.82) μg/L and 2.96 (2.21–4.73) μg/L at baseline and after 3 months, respectively (p = 0.07). After controlling for age, BMI and presentation of side effects, no association was demonstrated to the change in AMH levels (AMH2-AMH1).
Conclusions
Although menstruation of adolescent girls might be influenced by Covid-19 mRNA vaccine, it seems that the ovarian reserve estimated by AMH is not compromised.
Clinical trial registration
National Institutes of Health (NCT04748172).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
SARS-CoV-2 mRNA vaccination is associated with changes in menstrual patterns in adolescent girls, however, is not associated with a decrease in the ovarian reserve. |
Introduction
During the ongoing global Covid-19 pandemic, countries around the world have promoted vast vaccination programs to reduce morbidity and mortality [1, 2]. Although both are significantly lower in children than in adults, the risk of severe Covid-19 is not negligible, even among previously healthy children [3,4,5]. A large multicenter randomized control trial reported a favorable safety profile of the vaccine, a greater immune response than in young adults, and highly effectiveness against Covid-19 in children and adolescents aged 12–15 years. Based on these findings, on May 2021, the Food and Drug Administration (FDA) has authorized the expansion of Emergency Use Authorization for the BNT162b2 vaccine to include adolescents 12 to 15 years of age, with full approval of the vaccine in persons 16 years of age or older [6]. A month following the approval, Israel lunched national vaccination program for 12–16-year-old adolescences.
Despite the accessibility of vaccination services, a substantial minority of people delayed the acceptance or even refused to complete vaccination [7]. Vaccine hesitancy reflects parents’ concerns about the decision to vaccinate one’s children, and may lead to a reduction in vaccine coverage and eventually an increasing risk of infectious disease outbreaks and epidemics [8]. It is a significant barrier to achieve herd immunity in times of global pandemic, which is considered as one of the leading threats to public health [9, 10].
A special concern, that was spread quickly via the social media and had implications on the decision whether to undergo vaccinations, surrounded the concern whether SARS-CoV-2 mRNA vaccine could negatively influence future fertility, especially in young females [11]. Parents became hesitant and reluctant to get their adolescent girls to be vaccinated due to lack of confidence and evidence-based knowledge.
Studies reporting menstrual changes and abnormal bleeding patterns following vaccination are limited [12, 13]. A study from the Norwegian Institute of Public Health including cohort of 5688 Norwegian women reported heavier than normal bleeding as the change most associated with vaccination [12]. A recent study including 39,129 fully vaccinated women between 18 and 80 years old reported that 42% of women with regular menstrual cycles bled more heavily than usual after being vaccinated. Among women who typically do not menstruate, 71% on long-acting reversible contraceptives, 39% on gender-affirming hormones, and 66% of post-menopausal women reported breakthrough bleeding [14].
There is a paucity of data on the potential association between SARS-CoV-2 mRNA vaccine to future fertility. Recently, a study conducted by our group estimating the influence of vaccination on ovarian reserve did not find differences before and 3 months following first vaccination in the levels of serum anti-Mullerian hormone (AMH) [15] or the response to ovarian stimulation [30]. The results of these studies provided reassurance for women hesitant to complete vaccination against Covid-19 due to concerns regarding its effect on future fertility. Yet, these study included women 18 years old and above, and did not include data regarding gynecological well-being.
Due to all the aforementioned, and the limited published information regarding the sub-population of adolescence females, we aim to evaluate the possible effects of the mRNA SARS-CoV-2 vaccine on adolescence gynecological well-being before and 3 months following the first vaccination.
Materials and methods
This is a prospective study conducted at a university affiliated tertiary medical center, including adolescence females, aged 12 to 16 years, who were about to receive first vaccine by the Pfizer-BioNTech Covid-19 vaccine, between June and July 2021. Report of past Covid-19 infection confirmed by PCR test during infection or previous vaccination were causes for exclusion. As participates are under-aged, informed consent was signed by legal trustee.
Upon recruitment, all participants completed a computerized questionnaire about their general medical and gynecological background. Questions included information regarding the presence of secondary sexual characteristics, age of menarche, menstrual regularity (defined as periods that appear the same length every month with average of menstrual cycle length between 24 and 38 days), abnormal bleeding patterns (menorrhagia, inter-menstrual spotting), dysmenorrhea, sexual activity, contraception use and gynecological diagnosis (poly-cystic ovaries, endometriosis, ovarian cyst). In addition, blood samples for AMH plasma levels were collected. The second mRNA vaccine was given 21 days after the first. A follow-up visit was scheduled at 3 months after the first vaccination. During this visit, the participants were asked to complete a second computerized questionnaire focusing on their gynecological well-being and possible adverse effects following vaccinations. In addition, a second blood sample was collected for AMH levels.
Plasma concentrations of AMH were determined in the Sheba Medical Center accredited Endocrine Lab using Beckman Gen II ELISA kit with normal range values of 0.3–10.8 (mg/L) [16].
Primary outcome was defined as a change in menstrual regularity. Secondary outcomes included changes in menstrual intensity or length, side effects rate reported following the first and second shots and the estimated change in AMH levels at 3 months following the first vaccine minus the first AMH levels (Delta AMH = Second AMH − first AMH). Changes were also expressed as percentage changes (Delta AMH*100)/First AMH).
The study protocol was approved by the “Sheba Medical Center” Ethical Committee Review Board (ID 8121-21-SMC) on the 8th of February 2021 and was registered at the National Institutes of Health (NCT04748172).
Statistical analysis
Sample size calculation was performed for the primary outcome (change in irregularity rate; a priori analysis). For a two-tailed test, effect size = 0.5, α = 0.05 and 1-β = 0.80, a sample size of 35 was required.
We used paired t-test for comparing AMH level between first and second blood samples. We calculated the percent difference between the second and the first AMH values and defined a significant decline in AMH levels when the second AMH decreased by more than 10% than the first AMH. Comparisons between groups were conducted with Student’s t-test, Mann–Whitney U test, or Chi-square and Fisher’s exact tests as appropriate for normally distributed, not normally distributed or categorical variables respectively. Two-sided P < 0.05 was considered statistically significant. Logistic multivariate regression analysis was used to determine which factors were significantly and independently associated with the percent change in AMH levels.
Sample size calculation was performed with the G*Power 3.1. software. All additional statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.
Results
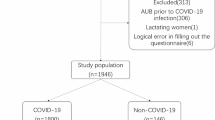
A total of 39 adolescent girls were recruited for the study, and of them, follow-up was completed by questionnaire and AMH sampling in 35 (90%) and 22 (56%), respectively. All participants completed two vaccinations (Fig. 1).
The clinical characteristics of the study population are presented in Table 1. The mean age was 13.8 (± SD 1.26) years. Mean BMI was 20.83 (± SD 5.44) with two girls being overweight and three obese (maximal BMI 33.59). Eight girls (22.9%) were pre-menarche. Regular menstruation was reported in 23 (65.7%) of the girls. None of the study population had known abnormal gynecological diagnoses, excluding two girls who were under investigation for endometriosis due to dysmenorrhea. All girls reported not being sexually active and none was exposed to hormonal contraception, nor any other contraception method.
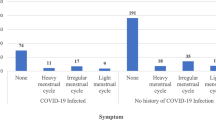
On follow-up 3 months after receiving the first vaccine, 4/8 (50%) of the pre-menarche girls reported experiencing their first menstruation. Among the girls reporting regular menstruation (n = 23), two (8.9%) suffered from irregularity during the first month following vaccination that has resolved, and in five (21.7%) girls the pattern has continued to be irregular on the 3-month follow-up visit. One girl reported longer bleeding duration than usual and one reported heavier bleeding. None experienced a reduction in the amount nor the length of menstrual bleeding.
Median AMH levels were 3.09 (IQR 1.96–4.82) μg/L and 2.96 (2.21–4.73 μg/L at baseline and after 3 months, respectively (p = 0.07). Figure 2 presents scatter plot of AMH levels at 3 months (y axis) as a function of the baseline AMH levels (x axis). The plot demonstrates that nearly all values in the study group are close to the diagonal line, reflecting no statistical difference for the change in AMH values at recruitment and at 3 months after vaccination.
Based on delta AMH (AMH3-AMH0), the percent change in AMH levels [(Delta AMH*100) AMH0] was calculated. Reduction (decreased by more than 10% than the first AMH), elevation (elevated by more than 10%) and no change were found in 9 (40.9%), 5 (22.7%) and 8 (36.4%) of the girls, respectively, with no statistical difference between the groups (p = 0.55). A logistic regression analysis was additionally performed to adjust for potential confounders. After controlling for age, BMI and side effects following the first and second vaccines, and in consistence with previous findings, no association was found to the change in AMH levels (AMH2-AMH1).
Table 2 describes the side effects following vaccination. The frequency of experiencing any side effect was higher by 1.5 following the second shot compared to the first (p = 0.016). The most frequent complain was local pain at the site of injection. Analysis evaluating whether an association between experiencing side effect to a possible change (more than 10% change) in AMH levels revealed no association following the first nor the second vaccine with p = 0.73 and p = 0.91, respectively.
Discussion
Principle findings
The main findings of our study are: (1) menstrual regularity was influenced by SARS-CoV-2 mRNA vaccination in adolescent girls. (2) SARS-CoV-2 mRNA vaccination might be associated with the appearance of menarche. (3) AMH was not significantly altered following vaccination. (4) No associations were found between the presence of side effects to the change in AMH levels nor the trend.
Clinical implications
The menstrual cycle is a complex interaction between various tissues, hormones, and organ systems. As such, the menstrual cycle is sensitive to endogenous and exogenous factors, including infection and changes in lifestyle. Emerging evidence suggests that SARS-CoV-2 infection, psychological stress related to the Covid-19 pandemic and Covid-19 vaccination, may all influence the menstrual cycle [13, 17,18,19].
Menstrual changes seems to be a part of the post-acute sequelae of SARS-CoV-2 following infection [17]. A retrospective study including 237 women of child-bearing age diagnosed with Covid-19 revealed that the average sex hormone concentrations and ovarian reserve did not change significantly and were comparable to age-matched controls [13]. However, nearly one-fifth of patients exhibited a menstrual volume decrease or cycle prolongation. The assumption is that those menstruation changes might be the consequence of transient sex hormone changes caused by suppression of ovarian function that quickly resumes after recovery.
The psychological stress related to the Covid-19 pandemic changes in menstruation has also been studied. Khan et al. [20] conducted a cross-sectional online survey study evaluating the menstrual cycle characteristics of 263 women in the reproductive phase of their lives during the Covid-19 pandemic in May 2020. They reported that elevated anxiety scores increased women’s menstrual symptoms while the length of periods and the number of pads used decreased. An anonymous digital survey conducted by Phelan et al. [19] including 1031 women revealed that 46% of the women reported a change in their menstrual cycle since the beginning of the pandemic, 53% reported worsening premenstrual symptoms, 18% reported new menorrhagia (p = 0.003) and 30% new dysmenorrhea (p < 0.0001) compared to before the pandemic.
Paucity of scientific research is published on menstrual changes following vaccination. Moreover, data regarding the sub-population of adolescent girls is lacking. Our study revealed that 20% (n = 7/35) of adolescent vaccinated girls will experience a change in menstrual regularity. 50% of premenarchal girls experienced their first menstruation during the 3-month follow-up. We acknowledge that this subgroup was relatively small; however, following this observation a possible association cannot be ruled out.
A few biological mechanisms plausibly explain the relationship between an acute immune challenge like a vaccine, that systematically affects hemostasis and induces inflammation process, and menstrual repair mechanisms [21, 22]. Menstruation has many of the features of an inflammatory process [23]. The complexity and sequence of inflammatory-type events, including several leukocyte types that change greatly through the menstrual cycle, are leading to the final tissue breakdown of the endometrium presented as bleeding. These leukocytes have a range of functions related to mucosal protection, breakdown, repair and remodeling [23]. Progesterone has anti-inflammatory properties, and its rapidly declining levels in the late secretory phase of each non-conception cycle, initiates a sequence of inflammatory events involving local inter-cellular interactions within the endometrium [21, 24]. Most probably, due to a similar mechanism evoking the immune system, menstrual irregularities have been reported with previous vaccines [25,26,27]. Hepatitis B studies have also indicated possible alternation in menstruation [27], and HPV post-market safety study found that over a quarter of participants reported temporary irregularities in menstruation [25, 26]. It might be reasonable to assume a similar inflammatory mechanism post-acute infection sequelae of SARS-CoV-2 [17] and vaccination.
Data on the influence of SARS-CoV-2 mRNA vaccinations on fertility and ovarian function are limited. The SARS-CoV-2 attacks human cells through binding of the viral S-protein to the ACE2 receptor. This S-protein is used in the mRNA vaccines as a presenting antigen and it was questioned whether such a pathway might negatively affect ovarian integrity [28, 29]. A recent study including 36 couples undergoing IVF treatment cycle before and 8–92 days after receiving mRNA SARS-CoV-2 vaccine found no influence of mRNA SARS-CoV-2 vaccine on patients’ performance during their immediate subsequent IVF cycle [30]. Bentov et al. demonstrate anti-SARS-CoV-2 IgG in follicular fluid (FF) from both infected and vaccinated IVF patients, with no evidence for compromised follicular function compared to controls [31]. A study conducted by our group estimating the ovarian reserve by anti-Mullerian hormone (AMH) did not find statistical difference before and 3 months following first vaccination in women above 18 years old [15]. Similar concerns to fertility were raised when Human Papilloma vaccine was introduced, especially as it was recommended for adolescents and young adults. These concerns were refuted by a population-based cohort study of nearly 200,000 women that found no association between the HPV vaccine and premature ovarian insufficiency [32]. Our study demonstrated that at 3 months after SARS-CoV-2 mRNA vaccinations, AMH levels did not change significantly. Our findings in adolescent girls are in concordance with previous studies demonstrating no significant influence on ovarian reserve following SARS-CoV-2 mRNA vaccination.
Limitation and strength
The study has strengths that should be acknowledged. To the best of our knowledge, this is the first study evaluating changes in menstrual patterns in the sub-population of adolescent girls following the new SARS-CoV-2 mRNA vaccine. Moreover, an objective parameter (AMH levels) was used in order to assess the potential influence of the vaccine on ovarian reserve. Each participant served as her own control. Follow-up was possible in 100% by questionnaire. All participants were diagnosed in a single medical center and were evaluated by the same team. AMH levels were determined in one central laboratory during the same time period and the two personal AMH evaluations were performed during the same laboratory run.
This study has also limitations that need to be mentioned. Although it was prospectively designed, for ethical reasons, we could not assign a priori a randomized unvaccinated control group. Study group was relatively small limiting the ability to generalize our findings. Although the questionnaire was completed by 100% of the participants, only 22 girls (62%) agreed to return for a second blood sampling at 3-month follow-up. The study examined plasma AMH levels at 3 months after the first vaccination. It could be argued that possible deleterious ovarian and AMH changes caused by the SARS-CoV-2 mRNA vaccinations might take effect only at a later time. Only long-term studies will be able to examine this issue.
Conclusion
In the present study, we found that menstrual patterns in adolescence girls were influenced by mRNA SARS-CoV-2 vaccinations. Moreover, it might have been associated to changes in the maturation of the endocrine system leading to menarche due to inflammatory process. Nevertheless, plasma AMH levels before and 3 months following two mRNA SARS-CoV-2 vaccinations did not change significantly in this population. Although changes have been observed in AMH levels on follow-up, non-have been found to be significant or related to the presence of side effects following vaccination.
Consultation and familiarity with this phenomenon’s should be reassuring for hesitant parents who are weighing their child’s pubertal development and future fertility against their risk of getting Covid-19. Changes to menstrual bleeding are not uncommon nor a sign to diminished future fertility, yet attention to these experiences is necessary to build trust in medicine.
Therefore, we conclude that SARS-CoV-2 mRNA vaccinations can be associated to changes in menstruation patterns in adolescent girls, however, are not associated with a decrease in ovarian reserve. This information could be of significant value to physicians and patients alike. Additional studies and longer term follow-up could further strengthen our findings.
Availability of data and materials
Data will be available upon request.
Abbreviations
- AMH:
-
Anti-Mullerian hormone
References
Billon-Denis E, Tournier JN (2020) COVID-19 and vaccination: a global disruption. Med Sci (Paris) 36(11):1034–1037
Tregoning JS, Brown ES, Cheeseman HM, Flight KE, Higham SL, Lemm NM et al (2020) Vaccines for COVID-19. Clin Exp Immunol 202(2):162–192
Stein M, Ashkenazi-Hoffnung L, Greenberg D, Dalal I, Livni G, Chapnick G et al (2022) The Burden of COVID-19 in children and its prevention by vaccination: a joint statement of the Israeli Pediatric Association and the Israeli Society for pediatric infectious diseases. Vaccines (Basel). 10(1):81
Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A et al (2020) SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 179(7):1029–1046
Delahoy MJ, Ujamaa D, Whitaker M, O’Halloran A, Anglin O, Burns E et al (2021) Hospitalizations associated with COVID-19 among children and adolescents—COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep 70(36):1255–1260
Frenck RW, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S et al (2021) Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 385(3):239–250
Salmon DA, Dudley MZ, Glanz JM, Omer SB (2015) Vaccine hesitancy: causes, consequences, and a call to action. Am J Prev Med 49(6 Suppl 4):S391–S398
Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J (2013) Vaccine hesitancy: an overview. Hum Vaccin Immunother 9(8):1763–1773
Sallam M (2021) COVID-19 vaccine hesitancy Worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel). 9(2):160
Xu Y, Zhang R, Zhou Z, Fan J, Liang J, Cai L et al (2021) Parental psychological distress and attitudes towards COVID-19 vaccination: A cross-sectional survey in Shenzhen. China J Affect Disord 292:552–558
Care H. [Available from: https://www.muhealth.org/our-stories/does-covid-19-vaccine-affect-fertility-heres-what-experts-say.
Trogstad L (2022) Increased occurrence of menstrual disturbances in 18- to 30-year-old women after covid-19 vaccination. SSRN Electron J. https://doi.org/10.2139/ssrn.3998180
Li K, Chen G, Hou H, Liao Q, Chen J, Bai H et al (2021) Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online 42(1):260–267
Katharine MN, Lee EJJ, Urooba A Fatima, Maria L Cox, Kathryn BH Clancy (2022) Characterizing menstrual bleeding changes occurring after SARS-CoV-2 vaccination View ORCID Profile
Mohr-Sasson A, Haas J, Abuhasira S, Sivan M, Amdurski HD, Dadon T et al (2021) The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum Reprod 37:534
Coulter B. [Available from: https://www.beckmancoulter.com/products/immunoassay/access-amh.
Carfì A, Bernabei R, Landi F, Group GAC-P-ACS (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–605
Demir O, Sal H, Comba C (2021) Triangle of COVID, anxiety and menstrual cycle. J Obstet Gynaecol 41(8):1257–1261
Phelan N, Behan LA, Owens L (2021) The impact of the COVID-19 Pandemic on Women’s Reproductive Health. Front Endocrinol (Lausanne) 12:642755
Khan SM, Shilen A, Heslin KM, Ishimwe P, Allen AM, Jacobs ET et al (2022) SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol 226(2):270–273
Evans J, Salamonsen LA (2012) Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord 13(4):277–288
Talaat KR, Halsey NA, Cox AB, Coles CL, Durbin AP, Ramakrishnan A et al (2018) Rapid changes in serum cytokines and chemokines in response to inactivated influenza vaccination. Influenza Other Respir Viruses 12(2):202–210
Berbic M, Fraser IS (2013) Immunology of normal and abnormal menstruation. Womens Health (Lond) 9(4):387–395
Berbic M, Ng CH, Fraser IS (2014) Inflammation and endometrial bleeding. Climacteric 17(Suppl 2):47–53
Gong L, Ji HH, Tang XW, Pan LY, Chen X, Jia YT (2020) Human papillomavirus vaccine-associated premature ovarian insufficiency and related adverse events: data mining of Vaccine Adverse Event Reporting System. Sci Rep 10(1):10762
Suzuki S, Hosono A (2018) No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: Results of the Nagoya study. Papillomavirus Res 5:96–103
Toshimitsu Shingu TU, Masuji Nishi, Kazuo Hayashida, Seizaburo Kashiwagi, Jun Hayashi, Masaro Kaji (1982) Menstrual abnormalities after hepatitis B vaccine. Kurume Med J 1982; 29:123–125
Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD et al (2007) The emerging role of ACE2 in physiology and disease. J Pathol 212(1):1–11
Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA (2011) Angiotensin-(1–7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril 95(1):176–181
Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas J, Nahum R, Aizer A (2021) Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod Biol Endocrinol 19(1):69
Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, Greenfield CS et al (2021) Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod 36(9):2506–2513
Naleway AL, Mittendorf KF, Irving SA, Henninger ML, Crane B, Smith N et al (2018) Primary ovarian insufficiency and adolescent vaccination. Pediatrics. https://doi.org/10.1542/peds.2018-0943
Acknowledgements
We acknowledge the Endocrine Laboratory Team of Sheba Medical Center for their cooperation.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
AM-S: conceptualization; formal analysis; investigation; methodology; project administration; roles/writing—original draft. Jigal Haas: conceptualization; formal analysis; investigation. MS: investigation. YZ: investigation. RH: investigation; methodology. RO: review and editing; methodology. AA: review and editing; methodology. JR: project administration; supervision; writing—review and editing; methodology; supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
The study protocol was approved by the “Sheba Medical Center” Ethical Committee Review Board (ID 8121-21-SMC) on the 8th of February 2021 and was registered at the National Institutes of Health (NCT04748172).
Consent to participate
Informed consent statement was requested by the ethical committee and signed by all participants or their legal apotropos.
Consent for publication
All the authors gave consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohr-Sasson, A., Haas, J., Sivan, M. et al. The effects of Covid-19 mRNA vaccine on adolescence gynecological well-being. Arch Gynecol Obstet 307, 1625–1631 (2023). https://doi.org/10.1007/s00404-023-06981-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-06981-2