Abstract
Objective
Misoprostol is a synthetic PGE1 analogue that is used for induction of labour. Current guidelines support the use of doses that do not exceed 25 mcg in order to limit maternal and neonatal adverse outcomes. The present meta-analysis investigates the efficacy and safety of oral compared to vaginally inserted misoprostol in terms of induction of labor and adverse peripartum outcomes.
Methods
We searched Medline, Scopus, the Cochrane Central Register of Controlled Trials CENTRAL, Google Scholar, and Clinicaltrials.gov databases from inception till April 2022. Randomized controlled trials that assessed the efficacy of oral misoprostol (per os or sublingual) compared to vaginally inserted misoprostol. Effect sizes were calculated in R. Sensitivity analysis was performed to evaluate the possibility of small study effects, p-hacking. Meta-regression and subgroup analysis according to the dose of misoprostol was also investigated. The methodological quality of the included studies was assessed by two independent reviewers using the risk of bias 2 tool. Quality of evidence for primary outcomes was evaluated under the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, ranging from very low to high.
Results
Overall, 57 studies were included that involved 10,975 parturient. Their risk of bias ranged between low-moderate. There were no differences among the routes of intake in terms of successful vaginal delivery within 24 h (RR 0.90, 95% CI 0.80) and cesarean section rates (RR 0.92, 95% CI 0.82, 1.04). Sublingual misoprostol was superior compared to vaginal misoprostol in reducing the interval from induction to delivery (MD – 1.11 h, 95% CI – 2.06, – 0.17). On the other hand, per os misoprostol was inferior compared to vaginal misoprostol in terms of this outcome (MD 3.45 h, 95% CI 1.85, 5.06). Maternal and neonatal morbidity was not affected by the route or dose of misoprostol.
Conclusion
The findings of our study suggest that oral misoprostol intake is equally safe to vaginal misoprostol in terms of inducing labor at term. Sublingual intake seems to outperform the per os and vaginal routes without increasing the accompanying morbidity. Increasing the dose of misoprostol does not seem to increase its efficacy.
Clinical trial registration
Open Science Framework (https://doi.org/10.17605/OSF.IO/V9JHF).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Current evidence indicates that oral (per os and sublingual) misoprostol intake is equal to vaginal misoprostol in terms of inducing labor as well as associated maternal and neonatal morbidity. |
Introduction
Induction of labour is used for decades in obstetrics; however, during the last years a steep rise in the percentage of deliveries that are medically induced is observed which from anecdotal reports seems to reach approximately 9.6% of all deliveries worldwide [1]. In the United States these rates are considerably higher reaching approximately 27%, while in Europe they seem to range between 6.8 and 33% [2, 3]. Whereas several methods have been used to induce cervical ripening as well as onset of labor, including oxytocin, prostaglandins, laminaria tents and foley balloon catheter, prostaglandins and oxytocin are the most widely accepted.
The American College of Obstetricians and Gynecologists suggests the use of prostaglandins for cervical ripening and labor induction at intervals that should be at least 3–6 h apart to avoid the risk of uterine tachysystole, although the minimum interval that is considered safe has not been standardized yet [4]. Regarding misoprostol, the World Health Organization recommends either oral (25 μg, 2-hourly) or vaginal route (25 μg, 6-hourly) for induction of labour [1]. In a previous meta-analysis that was conducted by Alfirevic et al., misoprostol combined with oxytocin and amniotomy has been proven to be the best method for achieving vaginal delivery within 24 h [5].
Misoprostol was originally licensed for oral use; however, vaginal and sublingual routes of administration are becoming more and more popular supported by pharmacokinetic studies focusing on the systemic bioavailability parameters achieved [6]. While the sublingual route seems to have the greater bioavailability, safety concerns have been raised from studies that were conducted in first trimester terminations [7].
Several articles have addressed the efficacy of the various routes of misoprostol in inducing a successful vaginal delivery and a previous meta-analysis that was published in 2008 suggested that both sublingual and vaginal routes seem to be comparable in terms of achieving vaginal delivery [8]. More recently, Alfirevic et al. published a systematic review comparing oral misoprostol to other methods of induction of labor (including oxytocin) and observed that it was associated with fewer cesarean sections [9]. Since then, several randomized trials have been published and an update is indicated to review current knowledge as, to date, there is no consensus on the optimal route of misoprostol intake for induction of labour.
The purpose of this meta-analysis is to investigate whether oral (either per os or sublingual) misoprostol intake is superior to vaginal administration in terms of inducing labor and leading to a vaginal delivery. Taking in mind the safety concerns that have been raised in the field of first trimester pregnancy complications, we also investigated its impact on maternal and neonatal morbidity outcomes.
Materials and methods
Protocol and registration
The present meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The study was based in aggregated data that have been already published in the international literature. Patient consent and institutional review board approval were not retrieved as they are not required in this type of studies. The study's protocol was published in open science framework, prior to the conduct of this review (Registration https://doi.org/10.17605/OSF.IO/V9JHF).
Eligibility criteria
Eligibility criteria for the inclusion of studies were predetermined. Randomized trials that compared the efficacy of oral (both per os or sublingual) to that of vaginal misoprostol in terms of induction of labour were considered eligible for inclusion. Quasi-randomized trials as well as observational studies (prospective and retrospective studies) were omitted from the systematic review. Only studies investigating induction of labor outcomes in singleton vertex presentations were included. Studies were included irrespective of the actual reason for induction of labour (antenatal pathology or prolonged pregnancy).
Information sources and search strategy
Two authors (V.P and M.P.) searched Medline (1966–2021), Scopus (2004–2021), Clinicaltrials.gov (2008–2021), EMBASE (1980–2021), Cochrane Central Register of Controlled Trials CENTRAL (1999–2021) and Google Scholar (2004–2021) along with the reference lists of electronically retrieved full-text papers. The date of the last search was set at April 30, 2022. No date restrictions were applied. Articles were limited to English language. The search strategy included the text words “induction; labour; misoprostol; oral; vaginal; sublingual; per os; oral” and is presented in brief in “Appendix”.
Studies were selected in three consecutive stages. Following deduplication, the titles and abstracts of all electronic articles were independently screened by three authors (V.P., M.P and T.K.) to assess their eligibility. The decision for inclusion of studies in the present meta-analysis was taken after retrieving and reviewing the full version of articles that were considered potentially eligible. Discrepancies that arose in this latter stage were resolved by consensus from all authors.
Study selection and data extraction
Outcome measures were predefined during the design of the present systematic review. Data extraction was performed using a modified data form that was based in Cochrane`s data collection form for intervention reviews for RCTs and non-RCTs [11].
The main outcomes of our study were the rates of successful vaginal delivery within 24 h, as well as cesarean section rates. Secondary outcomes included successful vaginal delivery beyond the limit of 24 h, interval to delivery, risk of uterine tachysystole, risk of postpartum hemorrhage and its accompanying side effects (risk of disseminated intravascular coagulation and need for obstetric hysterectomy) and neonatal side effects (including Apgar score values at 5` and need for admission to the NICU).
Assessment of risk of bias and quality of evidence
The methodological quality of the included studies was assessed by two independent reviewers using the risk of bias 2 (RoB 2) tool. RoB2 incorporates five domains that include assessment of (i) risk of bias that arises from the randomization process, (ii) risk of bias due to deviations from the intended interventions, (iii) missing outcome data, (iv) risk of bias in the measurement of the outcome, and (v) risk of bias in the selection of the reported result.
Quality of evidence was evaluated under the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, ranging from very low to high. More specifically, credibility of evidence will be assessed by taking into account the following domains: study limitations, directness, consistency, precision and publication bias. In particular, study limitations were evaluated based on risk of bias assessments (RoB 2 score), while directness was judged using the PICOS (population, intervention, comparison, outcome, study type) approach.
Synthesis of results
Statistical meta-analysis was performed with RStudio using the meta and metafor functions (RStudio Team (2015). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/). Statistical heterogeneity was not considered during the evaluation of the appropriate model of statistical analysis as the anticipated methodological heterogeneity of included studies did not leave space for assumption of comparable effect sizes among studies included in the meta-analysis [12]. Confidence intervals were set at 95%. We calculated pooled mean differences (MD) and 95% confidence intervals (CI) with the Hartung-Knapp-Sidik-Jonkman instead of the traditional Dersimonian-Laird random effects model analysis (REM). The decision to proceed with this type of analysis was taken after taking into consideration recent reports that support its superiority compared to the Dersimonian-Laird model when comparing studies of varying sample sizes and between-study heterogeneity [13]. When variables where expressed as median (range), median (interquartile range) or interquartile range and sample size transformation where performed to acquire the mean and standard deviation to include the studies in the meta-analysis [14].
Publication bias was assessed by examining the possibility of small-study effects through the visual inspection of funnel plots. The asymmetry of funnel plots was statistically evaluated using the Egger’s regression and Begg-Mazumdar’s rank correlation tests. The Duval and Tweedie`s trim and fill method was applied to impute missing effects irrespective of the asymmetry of the funnel plot.
Publication bias was evaluated by examining the potential presence of small-study effects through the visual inspection of funnel plots. Rücker’s Limit Meta-Analysis was applied to account for bias that arises due to small-study effects for primary outcomes. Outlier analysis was also undertaken to evaluate the effect of outlier studies on the overall effect size.
Prediction intervals
Prediction intervals (PI) were also calculated, using the meta function in RStudio, to evaluate the estimated effect that is expected to be seen by future studies in the field. The estimation of prediction intervals takes into account the inter-study variation of the results and express the existing heterogeneity at the same scale as the examined outcome.
Subgroup analysis
Subgroup analysis was designed following the retrieval of studies as several articles evaluated differences among sublingual and vaginal misoprostol as well as between per os and vaginal misoprostol; hence, oral misoprostol was subgrouped (as per os and sublingual) to evaluate for differences in summary effect estimates among the two methods.
Given the fact that in their previous meta-analysis Alfirevic et al. commented the lack of a substantial number of studies that could permit subgroup analysis based on the dose of misoprostol [9] in the present meta-analysis we performed meta-regression analysis of primary and secondary outcomes based on differences in misoprostol dosage (per dose administered and not total dose) among the two groups, to evaluate the potential effect of differences in the effect estimate. Subgroup analyses were also performed after arbitrary grouping of studies according to the maximum dose administered (Low dose = both groups received a dose of < 50 mcg of misoprostol, Intermediate = at least one of the two groups received = 50 < 100 mcg of misoprostol, High dose = at least one of the two groups received ≥ 100 mcg of misoprostol).
Results
Study selection and study characteristics
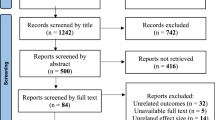
Following completion of the electronic search strategy we were able to identify 1436 potentially relevant articles. After reading the abstracts and, when needed, full texts we managed to limit them to an overall number of 69 articles of which we finally selected 57 randomized trials that involved 10,975 parturient [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. The methodological characteristics of included studies as well as patient characteristics are depicted in the “Appendix” and reveal comparable groups of oral vs vaginal misoprostol intake in terms of maternal age and body mass index, gestational age at delivery parity, Bishop score prior to the start of induction and neonatal birthweight.
Risk of bias of included studies
The evaluation of the methodological quality of included studies with the RoB2 tool revealed low risk of bias for the majority of studies, whereas some concerns were raised in 12 studies and high risk of bias was revealed in 9 studies (Fig. 1 and “Appendix”).
Synthesis of results
Overall, effect sizes of outcomes regarding per os and sublingual misoprostol compared to vaginal are presented in Table 1, while summary effect sizes along with subgroup analysis according to maximum misoprostol dose are presented in Table 2.
The various methods that were used were comparable in terms of successful vaginal delivery within 24 h (Fig. 2). Significant heterogeneity was observed among included studies. Small studies seem to influence the results and Rucker's meta-analysis revealed significant differences among the two groups in favor of the oral route (Table 3). Outlier analysis also indicated that several studies affected the overall effect with a p value closer to statistical significance for the adjusted estimate (p = 0.0857).
Forest plots of vaginal delivery within 24 h from onset of induction among parturient induced with oral misoprostol (subgrouped to sublingual and per os intake) and those induced with vaginally inserted misoprostol. (Vertical line = "no difference" point between the two groups. Blue squares = risk ratios; Diamonds = pooled risk ratios and 95 confidence intervals for all studies; Horizontal black lines = 95% CI; Horizontal red line = pooled 95% prediction intervals)
Cesarean section rates were comparable among the various routes of administration of misoprostol (Fig. 3). The dose of misoprostol did not influence these findings (RR 0.88, 95% CI 0.49, 1.60 for low doses, RR 0.94, 95% CI 0.81, 1.08 for intermediate doses and RR 0.89, 95% CI 0.75, 1.07 for high doses). Outliers, small study effects and meta-regression analysis did not influence the overall effect estimate.
Forest plots of risk of delivering with cesarean section among parturient induced with oral misoprostol (subgrouped to sublingual and per os intake) and those induced with vaginally inserted misoprostol. (Vertical line = "no difference" point between the two groups. Blue squares = risk ratios; Diamonds = pooled risk ratios and 95 confidence intervals for all studies; Horizontal black lines = 95% CI; Horizontal red line = pooled 95% prediction intervals)
Summary effect sizes of secondary outcomes are presented in Table 2. Briefly, the route of misoprostol intake did not influence delivery rates within 48 h from induction of labor or interval to delivery. Similarly, uterine tachysystole rates, risk of postpartum hemorrhage and risk of disseminated intravascular coagulation (DIC) were not affected by the route of administration. Concerning neonatal outcomes, risk of admission to the neonatal intensive care unit (NICU) was comparable among cases that received oral misoprostol to those that had intravaginal placement.
Of note, the interval to delivery was significantly reduced with sublingual misoprostol compared to vaginal (MD – 1.11 h, 95% CI – 2.06, – 0.17) and increased with per os misoprostol compared to vaginal (MD 3.45 h, 95% CI 1.85, 5.06) (Table 1).
Publication bias
The significance of the results of secondary outcomes were not altered by the trim-fill and small study effects (Rucker's) analysis. Contour enhanced funnel plots did not reveal significant bias with the exception of the uterine tachysystole outcome (“Appendix”). Trim and fill analysis did not reveal significant alterations in the level of statistical significance of results (Table 3).
Quality of evidence
The overall quality of the evidence for the evaluation of misoprostol's efficacy in successfully inducing labor and resulting in normal delivery was evaluated as high due to the low study limitations, very low possibility of indirectness and inconsistency of results, precision of findings and low risk of publication bias.
Discussion
Principal findings
The findings of our meta-analysis suggest that vaginal misoprostol increases the odds of successful vaginal delivery within 24 h compared to per os intake. However, sublingual intake might be superior to vaginal misoprostol. Of note, in terms of absolute time, sublingual misoprostol seems to be superior as it reduces the actual time from induction to delivery by approximately 1.11 h (compared to vaginal intake), whereas per os misoprostol seems to be the less effective mode as it increases this interval by approximately 3.45 h (compared to vaginal intake). However, direct comparison of these two modes of administration was not available in a substantial number of studies; hence, a network meta-analysis was not undertaken to evaluate the optimal route of intake.
Comparison with existing literature
A previous meta-analysis that investigated differences in labour outcomes among women receiving sublingual and vaginal misoprostol revealed increased rates of uterine tachysystole in the sublingual group; however, its findings were based in 5 studies [8], whereas our analysis included outcomes from 39 studies (10 of them regarding sublingual intake) and revealed that both oral (either per os or sublingual) and vaginal intake are equally safe in inducing labour. In 2001 Crane et al. in a retrospective cohort of 519 women observed that the incidence of tachysystole and hyperstimulation varied among the various routes of misoprostol intake [71]. The results of the present meta-analysis; however, seem to contradict this and leave plenty of space for decision making in clinical practice.
In 2014 Alfirevic et al. conducted a meta-analysis and concluded that the appropriate dose of misoprostol intake should be considered the one that ranges between 20 and 25 mcg and that it is preferable to use oral intake compared to vaginal, especially in the setting of developing countries in order to help reduce the risk of maternal and neonatal infection. Our meta-analysis did not reveal substantial differences among the three groups in terms of safety, however, it should be noted that between subgroups (Low, intermediate, high doses), substantial heterogeneity was noted that revealed a trend towards increased intervals form induction to delivery in higher and intermediate doses, whereas lower doses had a comparable effect estimate compared to vaginal misoprostol. To date, there is limited evidence on the actual impact of the vaginal route on maternal and neonatal infection rates (including chorioamnionitis, post-partum endometritis, neonatal infection and neonatal sepsis) and given the indirectness of reporting meta-analysis was not performed for these outcomes.
Strengths and limitations
The present meta-analysis is based in a large number of randomized controlled trials of low-moderate risk of bias. The secondary analyses that were performed permit safe interpretation concerning the accuracy of estimated effect sizes and estimation of risk of bias. Publication bias was thoroughly evaluated and indicated that our analysis is based mainly on studies of low-moderate risk of bias. Furthermore, for the first time it was found that the dose of misoprostol does not seem to affect rates of normal delivery (within 24- and 48-h).
On the other hand, several other confounders may partially limit the findings of this study, including co-administration of oxytocin for induction and augmentation of labor, parity, Bishop score at start of induction, presence of antenatal pathology (that may result in antepartum fetal distress, therefore, resulting in cesarean section) and gestational week at induction. However, meta-analyses that are based on aggregate data are always prone to these factors. In our analysis the Hartung-Knapp-Sidik-Jonkman random effects model was used which outperforms the traditional DerSimonian-Laird model as it consistently results in more adequate error rates when heterogeneous studies are considered for analysis; hence, confounders can be partially overlooked [13].
Conclusions and implications
Considering the results of the present meta-analysis it seems reasonable to conclude that sublingual misoprostol seems to offer better results in terms of a shorter interval to delivery and successful vaginal delivery within 24 h compared to vaginal misoprostol which seems to perform better than the per os intake (both as a pill or oral solution). Maternal and neonatal morbidity is not affected by either mode of intake. Intermediate doses can be acceptable according to the outcomes of the present study and seem to be the most efficacious in terms of shortening the interval to delivery.
Clinicians should be aware of these findings and could consider using misoprostol orally or sublingually instead of vaginally. Furthermore, there is need for future research, especially randomized controlled trials comparing oral and sublingual routes of administration as there is limited data on this direct comparison.
Data availability
The data that support this systematic review and meta-analysis are available from the corresponding author, upon reasonable request.
References
WHO Recommendations for Induction of Labour. Geneva: World Health Organization; 2011. 1, BACKGROUND. Available from: https://www.ncbi.nlm.nih.gov/books/NBK131965/.
Martin JA, Hamilton BE, Osterman MJK, Driscoll AK (2021) Births: final data for 2019. Natl Vital Stat Rep 70:1–51
European Perinatal Health Report (2010) Retrieved July 2021
ACOG Practice Bulletin No (2009) 107: Induction of labor. Obstet Gynecol 114:386–397
Alfirevic Z, Keeney E, Dowswell T, Welton NJ, Medley N, Dias S et al (2016) Which method is best for the induction of labour? A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess (Winch, Engl) 20:1–584
Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC (2002) Pharmacokinetics of different routes of administration of misoprostol. Human Reprod (Oxf, Engl) 17:332–336
Chong YS, Chua S, Arulkumaran S (2002) Sublingual misoprostol for first trimester termination of pregnancy: safety concerns. Human Reprod (Oxf, Engl) 17:2777
Souza AS, Amorim MM, Feitosa FE (2008) Comparison of sublingual versus vaginal misoprostol for the induction of labour: a systematic review. BJOG: Int J Obstet Gynaecol 115:1340–1349
Alfirevic Z, Aflaifel N, Weeks A (2014) Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014:CD001338-CD
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-34
Palas D, Ehlinger V, Alberge C, Truffert P, Kayem G, Goffinet F et al (2018) Efficacy of antenatal corticosteroids in preterm twins: the EPIPAGE-2 cohort study. BJOG: Int J Obstet Gynaecol 125:1164–1170
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1:97–111
IntHout J, Ioannidis JPA, Borm GF (2014) The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 14:25
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135
Agrawal A, Ramani B (2020) Sublingual versus vaginal misoprostol for induction of labour at term. IOSR J Dent Med Sci 19:16–19
Bansal M, Sharma I, Lagoo J, Jadhav H (2019) Sublingual versus vaginal use of misoprostol for induction of labor. Int J Reprod Contracept Obstet Gynecol 2019(8):6
Bartusevicius A, Barcaite E, Krikstolaitis R, Gintautas V, Nadisauskiene R (2006) Sublingual compared with vaginal misoprostol for labour induction at term: a randomised controlled trial. BJOG: Int J Obstet Gynaecol 113:1431–1437
Bennett KA, Butt K, Crane JM, Hutchens D, Young DC (1998) A masked randomized comparison of oral and vaginal administration of misoprostol for labor induction. Obstet Gynecol 92:481–486
Caliskan E, Bodur H, Ozeren S, Corakci A, Ozkan S, Yucesoy I (2005) Misoprostol 50 μg sublingually versus vaginally for labor induction at term: a randomized study. Gynecol Obstet Invest 59:155–161
Dadashaliha M, Fallah S, Mirzadeh M (2021) Cervical Versus Vaginal, and Sublingual Misoprostol for Labor Induction at Term Parturient: a Randomized Double-blind Clinical Trial.
Galidevara C, Chaturvedula L, Habeebullah S (2018) Comparison of oral, vaginal and sublingual misoprostol for induction of labour in premature rupture of membranes after 34 weeks of gestation: a randomized controlled trial. Int J Reprod Contracept Obstet Gynecol 7:1340
Gattás D, da Silva Junior JR, Souza ASR, Feitosa FE, de Amorim MMR (2018) Misoprostol administered sublingually at a dose of 12.5 μg versus vaginally at a dose of 25 μg for the induction of full-term labor: a randomized controlled trial protocol. Reprod Health 15:65
Hokkila E, Kruit H, Rahkonen L, Timonen S, Mattila M, Laatio L et al (2019) The efficacy of misoprostol vaginal insert compared with oral misoprostol in the induction of labor of nulliparous women: a randomized national multicenter trial. Acta Obstet Gynecol Scand 98:1032–1039
Ifariola D, Adeniyi AA, Adewara OE, Okere AR, Adebara IO, Bakare A et al (2020) Randomization of vaginal and sublingual misoprostol for cervical ripening and labor induction. Trop J Obstet Gynaecol 37:78–84
Jahromi BN, Poorgholam F, Yousefi G, Salarian L (2016) Sublingual versus vaginal misoprostol for the induction of labor at term: a randomized, triple-blind, placebo-controlled clinical trial. Iran J Med Sci 41:79–85
Khan OZ, Khan MH, Batool S, Akhtar R (2018) Comparing the efficacy of sublingual misoprostol and vaginal misoprostol for induction of labor at term live pregnancy. RMJ 43:444–447
Mehta RM, Patel BS, Shah AC, Jani SK, Patel VB, Patel AB et al (2020) A comparative study of vaginal misoprostol versus oral misoprostol for induction of labour. Int J Reprod Contracept Obstet Gynecol 9:2520–2524
Paungmora N, Herabutya Y, O-Prasertsawat P, Punyavachira P (2004) Comparison of oral and vaginal misoprostol for induction of labor at term: a randomized controlled trial. J Obstet Gynaecol Res 30:358–62
Rasheed R, Alam AA, Younus S, Raza F (2007) Oral versus vaginal misoprostol for labour induction. JPMA: J Pak Med Assoc 57:404–407
Sharami SH, Milani F, Faraji R, Bloukimoghadam K, Salamat F, Momenzadeh S et al (2014) Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Iran Med 17:652–656
Sheela CN, John C, Preethi R (2015) Comparison of the efficacy and safety of sublingual misoprostol with that of vaginal misoprostol for labour induction at term. J Obstet Gynaecol: J Inst Obstet Gynaecol 35:469–471
Sheir EM, El-Feky AE, El-Sayed AA (2019) Randomized controlled trial between sublingual and vaginal misoprostol for induction of labour at term. J Evid Based Women’s Health 9:407–15
Wallström T, Strandberg M, Gemzell-Danielsson K, Pilo C, Jarnbert-Pettersson H, Friman-Mathiasson M et al (2019) Slow-release vaginal insert of misoprostol versus orally administrated solution of misoprostol for the induction of labour in primiparous term pregnant women: a randomised controlled trial. BJOG: Int J Obstet Gynaecol 126:1148–55
Wing DA, Fassett MJ, Guberman C, Tran S, Parrish A, Guinn D (2004) A comparison of orally administered misoprostol to intravenous oxytocin for labor induction in women with favorable cervical examinations. Am J Obstet Gynecol 190:1689–94
Young DC, Delaney T, Armson BA, Fanning C (2020) Oral misoprostol, low dose vaginal misoprostol, and vaginal dinoprostone for labor induction: randomized controlled trial. PLoS One 15:e0227245
Zahran KM, Shahin AY, Abdellah MS, Elsayh KI (2009) Sublingual versus vaginal misoprostol for induction of labor at term: a randomized prospective placebo-controlled study. J Obstet Gynaecol Res 35:1054–1060
Souza ASR, Feitosa FEL, Costa AAR, Pereira APR, Carvalho AS, Paixão RM et al (2013) Titrated oral misoprostol solution versus vaginal misoprostol for labor induction. Int J Gynecol Obstet 123:207–212
Komala K, Reddy M, Quadri IJ, Suneetha B, Ramya V (2013) Comparative study of oral and vaginal misoprostol for induction of labour, maternal and foetal outcome. J Clin Diagn Res 7:2866–9
Ayaz A, Saeed S, Farooq MU, Ahmad I, Ali Bahoo ML, Saeed M (2009) Labour induction with randomized comparison of oral and intravaginal misoprostol in post date multigravida women. Malays J Med Sci 16:34–38
Shetty A, Danielian P, Templeton A (2001) A comparison of oral and vaginal misoprostol tablets in induction of labour at term. BJOG: Int J Obstet Gynaecol 108:238–243
Shetty A, Livingstone I, Acharya S, Rice P, Danielian P, Templeton A (2003) Oral misoprostol (100 microg) versus vaginal misoprostol (25 microg) in term labor induction: a randomized comparison. Acta Obstet Gynecol Scand 82:1103–1106
Adair CD, Weeks JW, Barrilleaux S, Edwards M, Burlison K, Lewis DF (1998) Oral or vaginal misoprostol administration for induction of labor: a randomized, double-blind trial. Obstet Gynecol 92:810–813
Wing DA, Park MR, Paul RH (2000) A randomized comparison of oral and intravaginal misoprostol for labor induction. Obstet Gynecol 95:905–908
Sharma DD, Chandnani KA (2019) Oral and vaginal route of misoprostol for induction of labour: a comparative study. Int J Reprod, Contracept, Obstet Gynecol 8(5):1956–63
Deshmukh VL, Yelikar KA, Waso V (2013) Comparative study of efficacy and safety of oral versus vaginal misoprostol for induction or labour. J Obstet Gynaecol India 63:321–324
Elhassan EM, Nasr AM, Adam I (2007) Sublingual compared with oral and vaginal misoprostol for labor induction. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet 97:153–154
Mehrotra S, Singh U, Gupta HP (2010) A prospective double blind study using oral versus vaginal misoprostol for labour induction. J Obstet Gynaecol: J Inst Obstet Gynaecol 30:461–464
Rahman H, Pradhan A, Kharka L, Renjhen P, Kar S, Dutta S (2013) Comparative evaluation of 50 microgram oral misoprostol and 25 microgram intravaginal misoprostol for induction of labour at term: a randomized trial. J Obstet Gynaecol Canada: JOGC 35:408–16
Adam I, Hassan OA, Elhassan EM (2005) Oral misoprostol vs. vaginal misoprostol for cervical ripening and labor induction. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet 89:142–3
Colón I, Clawson K, Hunter K, Druzin ML, Taslimi MM (2005) Prospective randomized clinical trial of inpatient cervical ripening with stepwise oral misoprostol vs vaginal misoprostol. Am J Obstet Gynecol 192:747–752
Kwon JS, Davies GA, Mackenzie VP (2001) A comparison of oral and vaginal misoprostol for induction of labour at term: a randomised trial. BJOG: Int J Obstet Gynaecol 108:23–26
Jindal P, Avasthi K, Kaur M (2011) A comparison of vaginal vs. oral misoprostol for induction of labor-double blind randomized trial. J Obstet Gynaecol India 61:538–42
Nopdonrattakoon L (2003) A comparison between intravaginal and oral misoprostol for labor induction: a randomized controlled trial. J Obstet Gynaecol Res 29:87–91
Toppozada MK, Anwar MY, Hassan HA, El-Gazaerly WS (1997) Oral or vaginal misoprostol for induction of labor. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet 56:135–9
Sultana NR, Rashid S, M, (2006) Oral versus vaginal misoprostol for induction of labour. J Bangladesh Coll Phys Surg 24:44–9
Ezechukwu PC, Ugwu EO, Obi SN, Chigbu CO (2015) Oral versus vaginal misoprostol for induction of labor in Enugu, Nigeria: a randomized controlled trial. Arch Gynecol Obstet 291:537–544
Prameela Sharma KD (2018) Comparison between use of oral misoprostol versus vaginal misoprostol for induction of labour at term. J Obstet Gynaecol India 68:88–92
Hall R, Duarte-Gardea M, Harlass F (2002) Oral versus vaginal misoprostol for labor induction. Obstet Gynecol 99:1044–1048
Paisarntantiwong R, Getgan M (2005) A comparison between single dose of 50 microg oral misoprostol and 25 microg vaginal misoprostol for labor induction. J Med Assoc Thailand Chotmaihet Thangphaet 88(2):S56-62
Carlan SJ, Bouldin S, Blust D, O’Brien WF (2001) Safety and efficacy of misoprostol orally and vaginally: a randomized trial. Obstet Gynecol 98:107–112
Pongsatha S, Vijittrawiwat A, Tongsong T (2005) A comparison of labor induction by oral and vaginal misoprostol. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet 88:140–141
Uludag S, Salihoglu Saricali F, Madazli R, Cepni I (2005) A comparison of oral and vaginal misoprostol for induction of labor. Eur J Obstet Gynecol Reprod Biol 122:57–60
Cheng SY, Ming H, Lee JC (2008) Titrated oral compared with vaginal misoprostol for labor induction: a randomized controlled trial. Obstet Gynecol 111:119–125
Fisher SA, Mackenzie VP, Davies GA (2001) Oral versus vaginal misoprostol for induction of labor: a double-blind randomized controlled trial. Am J Obstet Gynecol 185:906–910
How HY, Leaseburge L, Khoury JC, Siddiqi TA, Spinnato JA, Sibai BM (2001) A comparison of various routes and dosages of misoprostol for cervical ripening and the induction of labor. Am J Obstet Gynecol 185:911–915
le Roux PA, Olarogun JO, Penny J, Anthony J (2002) Oral and vaginal misoprostol compared with dinoprostone for induction of labor: a randomized controlled trial. Obstet Gynecol 99:201–205
Rizvi S, Umber F, Yusuf AW (2021) Labour induction at term; oral versus intravaginal misoprostol. Ann King Edward Med Univ 13:119–121
Schneider M, Ramsey R, Kao L, Bennett KA (2004) Misoprostol is effective for induction of labor in high risk pregnant women: a randomized controlled trial. Am J Obstet Gynecol 191:S73
Sheikher C, Suri N (2009) Comparative evaluation of oral misoprostol, vaginal misoprostol and intracervical Folley’s catheter for induction of labour at term. JK Science. 11:75–7
DebBarma AH, Baidya JL, Debasis R (2020) A comparative study of misoprostol oral versus vaginal route for induction of labour. Int J Reprod, Contracept, Obstet Gynecol 9:1907–13
Crane JM, Young DC, Butt KD, Bennett KA, Hutchens D (2001) Excessive uterine activity accompanying induced labor. Obstet Gynecol 97:926–931
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
VP and GD: conceived the idea, VP and MP: designed the project; TC, LVV, AK and AV tabulated data, VP and MP performed the statistical analysis and wrote the manuscript; VP and GD assessed bias among included studies; All authors: wrote the manuscript; VP and GD: supervised the project, wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Search plot

Risk of bias of included studies (RoB2 tool)

Primary outcomes (contour funnel plots, p-curves if available analysis exists)
Delivery within 24 h


Delivery with cesarean section


Secondary outcomes (contour funnel plots, p-curves if available analysis exists)
Interval to delivery (experimental = oral, control = vaginal)



Risk of uterine tachysystole (experimental = oral, control = vaginal)


Risk of postpartum hemorrhage (experimental = oral, control = vaginal)


Umbilical cord pH < 7.2

Risk of admission to the NICU (experimental = oral, control = vaginal)


Risk of meconium-stained amniotic fluid


Risk of 5-min Apgar score < 7


Delivery within 48 h

Outlier analyses
Delivery within 24 h

Cesarean section rates

Hours

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pergialiotis, V., Panagiotopoulos, M., Constantinou, T. et al. Efficacy and safety of oral and sublingual versus vaginal misoprostol for induction of labour: a systematic review and meta-analysis. Arch Gynecol Obstet 308, 727–775 (2023). https://doi.org/10.1007/s00404-022-06867-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06867-9