Abstract
Purpose
We aimed to determine the predictive values of fetal pancreas size and maternal serum biomarkers glycated albumin (GA) and insulin-regulated aminopeptidase (IRAP) for gestational diabetes mellitus (GDM).
Materials and methods
In this prospective observational study including 109 pregnant women, the fetal pancreas size and maternal serum biomarkers GA and IRAP were measured at the gestational age of 20–22 weeks and later at the gestational age of 24–28 weeks, in 19 participants of them, GDM was confirmed with the 75-g oral glucose tolerance test (OGTT) and the fetal pancreas size was measured in all the participants again.
Results
The median fetal pancreas sizes were significantly higher in women with or without GDM when measured at the 24–28 weeks of pregnancy compared to those at the 20–22 weeks of pregnancy (p < 0.05). At both of the 20–22 and 24–28 weeks of pregnancy, the median values of fetal pancreas sizes in the women with or without GDM were found comparable (p > 0.05). There were no significant differences between pregnant women with or without GDM regarding maternal serum biomarkers GA and IRAP (p > 0.05). Multivariate logistic regression analysis revealed no meaningful association of study parameters with the development of GDM.
Conclusion
The fetal pancreas size and maternal serum biomarkers GA and IRAP provide no potential for early prediction of GDM at the 20–22 weeks of gestation. Further studies, including serial measurement of these parameters during the second and third trimesters of GDM pregnancies, may clarify their role in the antenatal care of women with GDM.
Clinical trials
NCT05392231.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ultrasonographic evaluation of the fetal pancreas size and measurements of maternal serum glycated albumin and insulin-regulated aminopeptidase performed at the 20–22 weeks of gestation do not provide a potential to be used as predictors for early detection of gestational diabetes mellitus. |
Introduction
Gestational diabetes mellitus (GDM) is still an important health condition that is first recognized in pregnancy with a prevalence of 7–9% [1]. GDM continues to be a hot research subject in perinatology due to its adverse effects developed in a short or long period on mothers and babies from neonatal to adult life [2]. In GDM mothers, mild–moderate degree hyperglycemia may not be life-threatening during the current pregnancy, however, their fetuses can develop fetal hyperinsulinemia and macrosomia, and neonatal hypoglycemia. There are piling data clarifying the importance of exposure of fetuses to an intrauterine hyperglycemic environment that may also lead to chronic health conditions later in life [3, 4].
Fetuses of GDM mothers could have hyperglycemia for a while before their diagnosis and hyperglycemia may affect them during the second trimester, and insulin therapy in their mothers may not be successful as expected for the prevention of long-term metabolic disorders during their adulthood. Therefore, a standard detection of GDM at the 24–28 weeks of gestation might be somewhat late for therapeutic interventions and could not resolve all the adverse effects of intrauterine hyperglycemic exposure on the babies. There is a requirement to develop novel screening modalities to be aware of high-risk women for GDM in the second trimester and to have an earlier opportunity for appropriate treatments before a routine GDM diagnosis [4]. That can be of great significance for the early prediction and diagnosis of GDM.
Although the specific causes and mechanisms of GDM development are considerably unclear arsenic exposure [5] and COVID-19 [6], as well as the role of maternal obesity and insulin resistance [7, 8] and the interaction of obesity and inflammation [9], are among the proposed factors. With ongoing research activities, some novel ultrasonographic findings and blood biomarkers have been reported as potential predictions of GDM, including angiopoietin-like protein 8 [10], plasma fatty acid-binding protein 4 [11], adiponectin [12], leptin, lipocalin-2, and plasminogen activator inhibitor-2 [13], fetuin-A, N-terminal pro-atrial natriuretic peptide [14], but the low availability of these biomarkers in clinical settings limits their application. The clinical implications of previous research activities have not found a place in the expected level clinical settings for the prediction and diagnosis of GDM. This leads to the continuation of new research to understand the pathogenesis of GDM and to seek reliable and successful tests for the prediction and diagnosis of GDM. By reviewing the current perinatology literature, it has been noted that it may be advantageous to investigate fetal pancreas size and serum biomarkers glycated albumin (GA) and insulin-regulated aminopeptidase (IRAP) together in pregnancies with GDM.
Imaging of the fetal pancreas has been under investigation since the 1980s. In early studies, the feasibility of its imaging during fetal life and the technique of imaging the pancreas, and the measurement of its size were topics of studies. Later, the value of its measurement in women with GDM and the possibility of using reference sizes related to the fetal pancreas were assessed [15,16,17,18,19,20,21].
Taken together, nowadays, there are several recommended criteria or models to use for the prediction of GDM that consist of a variety of sociodemographic characteristics, serological biomarkers, and ultrasonographic parameters; however, they cannot always be as successful as expected to obtain reliable results in perinatology practice [1, 22]. The early determination of GDM may help to care for GDM mothers earlier than usual clinical diagnosis and improve perinatal outcomes. Considering these aspects of the topic, there is a need for studies dealing with the determination of successful tests to predict the future development of GDM. The current study aimed to assess the clinical values of ultrasonographic measurement of fetal pancreas size and the serum biomarkers GA and IRAP for earlier detection of GDM in pregnant women at the gestational age of 20–22 weeks and to shed light on the possibility of earlier detection of GDM with a scientific basis in a population of pregnant women without a higher risk of GDM.
Materials and methods
Study groups
With a design of prospective observational study, this research was carried out in the Perinatology Clinic of Haseki Training and Research Hospital, affiliated with the University of Health Sciences, in Istanbul, between February 2022 and June 2022, following the approval of the local ethics committee (date: February 09, 2022, Registry No: 05-2022) and the valid Helsinki Declaration. After all the objectives of this work and the context of all the procedures that could be undertaken were properly explained to each pregnant woman, the participant was requested to sign an informed written consent form. The study was registered on Clinical trials (NCT05392231).
Eligible pregnant women with a maternal age of between 18 and 42 years and at 20–22 weeks of gestation were consecutively included in this study during their first antenatal visit. Then, during the second antenatal visit, at 24–28 weeks of pregnancy, a maternal 75-g, 2-h OGTT was performed and according to its results, the study participants were divided into the normal glucose tolerance (NGT) group, consisting of healthy pregnant women who did not develop GDM, and the GDM group, consisting of pregnant women with GDM. The exclusion criteria were multiple pregnancies, pre-gestational diabetes, fetal congenital malformations, placental and amniotic fluid abnormalities, preeclampsia, severe systemic disease, and long-term systemic drug use. For the diagnosis of GDM, there is a need for at least one of the following three criteria of 75-g, 2-h OGTT thresholds that were met or found with a higher value: fasting 92 mg/dL, 1-h 180 mg/dL, or 2 h 153 mg/dL at 24–28 weeks of gestation [23].
During the first antenatal visit, at 20–22 weeks of pregnancy, the following procedures were performed: the collection of maternal baseline clinical characteristics and routine biochemistry tests; an ultrasound imaging for fetal anatomy and growth and the measurement of fetal pancreas size; and measurement of maternal serum levels of GA and IRAP with the help of a standard biochemical analyzer. The second antenatal visit, at 24–28 weeks of pregnancy, included an ultrasound examination of fetal pancreas size measurement.
The maternal age, gravidity and parity, status of education as literate, primary to high school, or higher education, being native or emigrant, family history of diabetes mellitus (DM), history of GDM, smoking, mode of conception, body mass index prenatally, weight gain during pregnancy, the use of pharmacological treatment in the GDM women as dietary arrangement or insulin use, mode of delivery as vaginal or cesarean, and fetal gender, and gestational age at the first and second antenatal visits were collected from the electronic medical records of the participants.
Ultrasound examination of fetal pancreas
All pregnant women underwent a 2D ultrasound scan with a 1–7 MHz probe. The fetal pancreas size of each fetus was measured twice throughout pregnancy at the 20–22 and 24–28 weeks of pregnancy, by a professional perinatologist (FYG). For these measurements, intra-observer reliability was examined with Kappa statistics that provided Kappa coefficient ranged from 0.83 to 0.89 and it was interpreted as strong agreement. To determine fetal pancreas size, the circumference of the fetal pancreas was measured using the trace method in accordance with the previously published method [19]. In brief, the fetal pancreas was scanned by slightly rotating and angling the transducer caudally from the transverse plane of the fetal abdominal circumference. It was determined that the location of the fetal spine between 3 and 5 o’clock or 7 to 9 o’clock was optimal for a good quality visualization of the fetal pancreas. The view of the fetal pancreas was explained as an elongated, echogenic structure that is located among the fetal stomach, aorta, and vertebral spine. The circumference was measured using the free-hand tracing function after freezing the image from the left caudal edge to the right ventral edge (Fig. 1). In comparison to the fetal liver, the pancreas had a slightly hyperechoic view. Each measurement was done two or three times in each of the fetuses, with the average size used to calculate the final measurement.
Blood collection and analysis
Fasting blood samples were collected from each study participant when they attended the first and second antenatal visits at 20–22 and 24–28 weeks of pregnancy for routine laboratory tests. The studied biomarkers, GA and IRAP were measured from the blood samples at the 20–22 weeks of pregnancy Maternal biochemical tests were performed. The blood samples taken for the study were centrifuged at 1000 xg for 10 min, separated into secondary tubes, and stored at − 50 °C. Serum GA and IRAP concentrations were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (ELK Biotechnology, P.R.C.) according to the manufacturer’s protocol. Serum samples were not diluted. The standards were serially diluted from starting concentrations of 1000 pmol/mL down to 15.63 pmol/mL for GA and from 10 ng/mL to 0.16 ng/mL for IRAP, in the standard diluent supplied with the kit. The intra- and inter-assay coefficients of variance varied between 8 and 10% for the tests.
Statistical analysis
To perform the descriptive and analytic evaluation of study data, the IBM SPSS v25 (IBM SPSS, Armonk, NY, United States) was used. Graphical figures were designed with the GraphPad Prism v9 (Graphpad, San Diego, CA, USA). The numerical data as mean with standard deviation, median with interquartile range, or count with percentage were presented as appropriate. After examining the normality of numerical data with the Kolmogorov–Smirnov test, t, Mann–Whitney, or Wilcoxon signed-rank test were used as appropriate. To determine the significance of categorical data, the chi-square test was used. We performed a logistic regression analysis to test whether the values of study variables including the fetal pancreas size and serum biomarkers GA and IRAP at the 20–22 weeks of pregnancy were associated with the development of GDM. Furthermore, after the selection of adequate variables for the logistic regression model, we performed a multivariate logistic regression analysis to determine the independence and contribution of the study variables to the development of GDM. If the p value was below 0.05, differences were considered significant.
Results
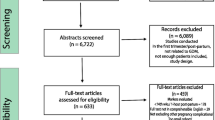
A flow chart of the study population is shown in Fig. 2. A total of 90 women with NGT and 19 women with GDM were included for analyses of study variables. The baseline clinical parameters of the NGT and GDM groups are listed in Table 1. The women in the GDM group show higher pre-gestational BMI, age, gravidity, parity, family history of DM, and obstetric history of GDM than in the NGT group (p < 0.05). There were no significant differences in terms of the ratios of ethnicity, education level, smoking status, modes of conception and delivery, gestational weight gain, and the ratio of female fetuses between the NGT and GDM groups (p > 0.05). The gestational age at the first and second antenatal visits ranged from 20 to 22 and 24–28 weeks of pregnancy, respectively in both of the study groups. The ratio of insulin treatment was 21.1% in the GDM group.
Table 2 shows the maternal laboratory findings of the NGT and GDM groups. The values of fasting and 1- and 2-h 75-g OGTT, glycated hemoglobin (HbA1c), homeostatic model assessment of insulin resistance (HOMA-IR), and triglyceride were significantly higher than those of the NGT group (p < 0.05). The values of serum creatinine, aspartate aminotransferase, alanine aminotransferase, albumin, lactate dehydrogenase, and low-density lipoprotein cholesterol were found as similar between the study groups (p > 0.05). There was a significantly lower mean value of high-density lipoprotein cholesterol (HDL-C) in the GDM group than that in the NGT group (p < 0.05).
Figure 3 displays the median values of fetal pancreas size in women with NGT and GDM at the 20–22 and 24–28 weeks of pregnancy. Statistical analyses revealed significantly higher median values of fetal pancreas size measured in the women with NGT and GDM at the 24–28 weeks of pregnancy compared to those at the 20–22 weeks of pregnancy [NGT: 7.5 (7.1–8.3) vs. 5.9 (5.6–6.6), p < 0.05; GDM: 7.6 (7.2–9.1) vs. 6 (5.6–6.8), p < 0.05]. At both of the 20–22 and 24–28 weeks of pregnancy, the median values of fetal pancreas size in the NGT and GDM groups were found comparable [20–22 weeks of pregnancy: 5.9 (5.6–6.6) and 6 (5.6–6.8), respectively; p > 0.05, and 24–28 weeks of pregnancy: 7.5 (7.1–8.3) and 7.6 (7.2–9.1), respectively; p > 0.05].
Median values of fetal pancreas size in women with NGT and GDM at the 20–22 and 24–28 weeks of pregnancy. Data are expressed as median with interquartile range (25–75%). Mann–Whitney test revealed significantly higher median values of fetal pancreas size measured in the women with NGT and GDM at the 24–28 weeks of pregnancy compared to those at the 20–22 weeks of pregnancy (p < 0.05). There was no significant difference between the study groups regarding the median value of fetal pancreas size measured at the 20–22 and 24–28 weeks of pregnancy (p > 0.05). NS not significant, NGT normal glucose tolerance, GDM gestational diabetes mellitus
Figure 4 shows the median values of serum GA in the women with NGT and GDM at the 20–22 weeks of pregnancy. We found that the median values of serum GA in the women with NGT and GDM were found comparable [959.8 pmol/mL (789.3–1036.0) and 933.4 pmol/mL (870.7–1038.0), respectively; p > 0.05].
Median values of serum GA in women with NGT and GDM at the 20–22 weeks of pregnancy. Data are expressed as median with interquartile range (25–75%). Mann–Whitney test revealed no significant difference between the study groups (p > 0.05). GA glycated albumin, ns not significant, NGT normal glucose tolerance, GDM gestational diabetes mellitus
Figure 5 represents the median values of serum IRAP in the women with NGT and GDM at the 20–22 weeks of pregnancy. We found that the median values of serum IRAP in the women with NGT and GDM were found comparable [0.06 ng/mL (0.04–0.10) and 0.06 ng/mL (0.04–0.085), respectively; p > 0.05].
Median values of serum IRAP in women with NGT and GDM at the 20–22 weeks of pregnancy. Data are expressed as median with interquartile range (25–75%). Mann–Whitney test revealed no significant difference between the study groups (p > 0.05). IRAP, insulin-regulated aminopeptidase; ns not significant, NGT normal glucose tolerance, GDM gestational diabetes mellitus
With the multivariate logistic regression analysis, the fetal pancreas size and serum biomarkers GA and IRAP revealed no significant effect (p = 0.21, p = 0.51, and p = 0.07; respectively) on GMD development in a model that included other clinical variables, such as the maternal age, gravidity, family history of DM, history of GDM, pre-pregnancy BMI, HbA1c, HOMA-IR, triglyceride, and HDL-C if they presented a p value of < 0.05 with the univariate logistic regression analysis.
Discussion
In terms of baseline clinical and laboratory parameters collected in the study population, overall, women with GDM had no meaningful features that can affect the state of maternal and fetal health and make an important contribution to the status of fetal pancreas size and maternal serum biomarkers GA and IRAP. Women with or without GDM had similar values of fetal pancreas size measured earlier than and during the period of maternal GDM screening. The reason for the significant difference between fetal pancreas measurements at 20–22 and 24–28 weeks of gestation in both populations was interpreted as the growth of the fetus. In this study, when the potential of using fetal pancreas size with the maternal serum GA and IRAP biomarkers measured together at the 20–22 weeks of gestation in the prediction of GDM was examined, it was seen that the prediction abilities of these biomarkers were not as expected as analyzed with univariate and multivariate logistic regression tests.
There had been disagreements among countries about how to screen and diagnose the status of glucose tolerance during pregnancy. There were not enough data to support universal pregnancy screening. Consequently, pregnant women with obesity, a family history of DM, a history of GDM, or a baby with weight of > 4.5 kg should be provided screening with the two-hour OGTT during their scheduling visit [24]. Early detection and appropriate management of GDM including pharmacological measures has a potential to improve the outcome as summarized in a recent publication [25]. To develop novel strategies regarding the early prediction of GDM, new models with maternal variables [26], ultrasound parameters including fetal heart rate [27, 28] and Doppler ultrasound findings of the maternal uterine artery, and biochemical parameters [13] were investigated.
There are some important concepts that should be considered to better understand the background of GDM. First, pregnancies complicated by GDM are characterized by increased placental expression of VEGF and CD31, and the expression of these markers is also independently associated with maternal increased pre-gestational BMI and gestational weight gain [9]. Second, triglyceride concentrations had significant importance for the risk of GDM [8], whereas lipid profile was correlated to LGA and macrosomia [29]. Finally, diagnosis of hypertensive disorders, perinatal mortality, obesity, and macrosomia increase the risk of GDM in subsequent pregnancies [7].
When the studies on ultrasonographic evaluation of the fetal pancreas in the literature are examined, the viewability of the fetal pancreas and imaging standards are prioritized in early studies. In current studies, with the increasing success in imaging the fetal pancreas, issues related to the clinical prediction, diagnosis, and outcome of fetal pancreas evaluation in pregnancies with diseases such as DM have been included. Advances in ultrasonography devices have also significantly increased the success and reliability of fetal pancreas evaluations. There is no consensus yet on the status of fetal pancreatic enlargement in diabetic pregnancies. Our study examined the size of the fetal pancreas in cases with GDM. Fetal pancreas size was measured twice at 20–22 and 24–28 weeks of pregnancy to understand its relationship with the process of GDM development and progress.
In the 1980s, there were few articles that described the ultrasound imaging of the fetal pancreas [15, 16]. In one of those studies, the fetal pancreas was assessed with focused imaging of the fetal abdomen to detect the pancreas and the fetal stomach [17]. In their study, the pancreas could be observed in 77 of the 149 patients (51.7%) and menstrual age and fetal position were found to be important factors in predicting the frequency of pancreatic visibility. Hata et al. assessed the fetal pancreas after 20 weeks of pregnancy and found that it could be measured properly in 80% of the instances [18]. They found that the length of the fetal pancreas was also found to be related to gestational age. The authors noted that fetal biometry exposed a variety of fetal organs; consequently, it was essential to have clear images and landmarks.
Kivilevitch et al. conducted a study to determine the sonographic feasibility of monitoring the fetal pancreas and its normal development in fetuses between 19 and 35 weeks of gestation [19]. They determined the typical reference range for fetal pancreas circumference during pregnancy, and the total acceptable visualization rate was 61.6%. Hatice et al. discovered that the morphology of the pancreas, as well as its hyper-echogenicity, were both substantially and positively linked with the risk of GDM [20]. They noted that the hyper-echogenic pancreas was strongly and positively linked to an increased risk of GDM.
Another study performed by Gilboa et al. investigated the effect of glycemic control treatment on GDM and pre-gestational diabetes, the effect of the intervention was examined on the size of the fetal pancreas [21]. Their study population included women with pre-gestational diabetes getting insulin therapy or GDM receiving insulin or oral hypoglycemic therapy between 19 and 36 weeks of gestation. They measured pancreatic circumference and compared it to the typical reference range. They highlighted a relationship between glycemic control therapy, pancreatic size, and gestational age. In light of the existing literature, as mentioned above, data are supporting that fetal pancreas size increases by gestational age and shows a significant relationship with GDM status. Although these results suggest that fetal pancreas size measurement may be valuable for the early prediction of GDM, our current findings do not support this opinion.
HbA1c is the standard test for long-term glucose monitoring and is associated with chronic diabetic consequences [30]. However, in clinical conditions when hemoglobin metabolism may be hampered, such as hemolytic, secondary, or iron deficiency anemia, hemoglobinopathies, and pregnancy, HbA1c is not advocated [31]. GA is a test that assesses short-term glycemia and is unaffected by circumstances that falsely alter HbA1c results [32]. While HbA1c reflects glucose control from 2 to 3 months prior, GA shows the status of glycemic control over 2–3 weeks [33]. Zhu et al. conducted a study to assess the diagnostic performance of HbA1c, GA, and fasting plasma glucose in diagnosing GDM [34]. They found that fasting plasma glucose had a higher diagnostic value than GA and HbA1c for detecting GDM at 24–28 weeks of pregnancy and that the diagnostic values of fasting plasma glucose, GA, and HbA1c were similar for gestational ages less than or more than this range. Chume et al. investigated the clinical value of GA in the diagnosis of GDM in a study [35]. Their findings revealed that GA has low overall accuracy in diagnosing GDM and is unable to effectively differentiate between women with and without GDM. In accordance with those findings, the GA data of our study did not support the clinical value of maternal serum for the prediction of GDM.
IRAP is related to zinc-dependent membrane aminopeptidases and is expressed in a variety of cell types, especially in two well-known tissues, muscle, and fat [36]. In addition to glucose transporter type 4 (GLUT4), IRAP can be used as a surrogate measure of insulin-regulated vesicular traffic since its presence is necessary for sustaining proper insulin-dependent molecular transport [37]. Those with type 2 DM have impaired insulin-stimulated transfer of IRAP to the cellular surface of muscle and fat tissues. This deficiency may result in diminished metabolism of IRAP and, consequently, an increase in the activity of peptide hormones that are substrates for IRAP. In a recent study, Mostafa et al. investigated the role of the extracellular part of IRAP in the development of insulin resistance in patients with type 2 DM [38]. They revealed that IRAP levels were significantly lower in the diabetic population compared to the healthy population, and in addition, they hypothesized that IRAP may be a helpful and direct marker for insulin resistance detection in the diabetic population. Tian et al. conducted a prospective study to determine the relationship between serum concentrations of IRAP and hypertensive diseases in pregnancy, GDM, and perinatal mortality [39]. They concluded that serum IRAP levels were significantly lower in patients with hypertensive disorders and GDM, and were exceedingly low in patients with fetal death compared to healthy pregnant women. Overall, considering the current studies and our findings, there is a need for further studies investigating the role of serum IRAP concentration in healthy and GDM conditions.
There are limitations to this study. First, during determining the sample size of the study, a convenient sampling was chosen based upon the absence of previous relevant studies on this topic. This is a limitation since it prevents understanding of the statistical power of the study. Second, serial measurements of ultrasonographic and serum biomarkers could be helpful to understand the trends of their changes during healthy and GDM pregnancies. Third, GDM pregnancies could be classified according to the intervention modes of diabetes as nutritional regulation or insulin usage. However, first time in the literature, the fetal pancreas size was evaluated for its ability to predict GDM before the usual period of GDM screening. In this study, considering the pancreatic size evaluation and performing other routine antenatal care procedures, we prepared the 20–22 weeks of gestation as the convenient time. In addition, the maternal serum biomarkers GA and IRAP were measured in the same clinical setting, to improve their predictive success. However, current data did not support their concomitant work as laboratory tests of GDM management. Much effort is needed before their contributions to the development of GDM could be benefited in diagnostic and therapeutic interventions of GDM.
In conclusion, the fetal pancreas size and maternal serum biomarkers GA and IRAP do not provide a potential for early prediction of GDM at the 20–22 weeks of gestation. Considering the measurement of fetal pancreas size during antenatal care of pregnant women, further studies are needed to shed light on its role in the development of the fetus and its exposure to diabetic influences. Since the proteins GA and IRAP may involve in several cellular functions in the context of diabetic status, their roles in the development and progress of GDM wait for clinical and basic science research activities. The clinical advantages of adding first-trimester test candidates to these experimental measurements for the prediction of GDM also need to be addressed in future research.
References
Man B, Schwartz A, Pugach O, Xia Y, Gerber B (2021) A clinical diabetes risk prediction model for prediabetic women with prior gestational diabetes. PLoS One 16:e0252501–e0252501. https://doi.org/10.1371/journal.pone.0252501
Shou C, Wei Y-M, Wang C, Yang H-X (2019) Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Matern Fetal Med 1:91–94
Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY et al (2017) In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 40:679–686. https://doi.org/10.2337/dc16-2397
Wu Y-T, Zhang C-J, Mol BW, Kawai A, Li C, Chen L et al (2021) Early prediction of gestational diabetes mellitus in the Chinese population via advanced machine learning. J Clin Endocrinol Metab 106:e1191–e1205. https://doi.org/10.1210/clinem/dgaa899
Salmeri N, Villanacci R, Ottolina J, Bartiromo L, Cavoretto P, Dolci C et al (2020) Maternal arsenic exposure and gestational diabetes: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu12103094
Eskenazi B, Rauch S, Iurlaro E, Gunier RB, Rego A, Gravett MG et al (2022) Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study. Am J Obstet Gynecol 227:74.e1-74.e16. https://doi.org/10.1016/j.ajog.2021.12.032
Yoles I, Sheiner E, Wainstock T (2021) First pregnancy risk factors and future gestational diabetes mellitus. Arch Gynecol Obstet 304:929–934. https://doi.org/10.1007/s00404-021-06024-8
Grieger JA, Leemaqz SY, Knight EJ, Grzeskowiak LE, McCowan LM, Dekker GA et al (2022) Relative importance of metabolic syndrome components for developing gestational diabetes. Arch Gynecol Obstet 305:995–1002. https://doi.org/10.1007/s00404-021-06279-1
Sirico A, Rossi ED, Degennaro VA, Arena V, Rizzi A, Tartaglione L et al (2022) Placental diabesity: placental VEGF and CD31 expression according to pregestational BMI and gestational weight gain in women with gestational diabetes. Arch Gynecol Obstet. https://doi.org/10.1007/s00404-022-06673-3
Leong I (2018) ANGPTL8 as an early predictor of gestational diabetes mellitus. Nat Rev Endocrinol 14:64. https://doi.org/10.1038/nrendo.2017.167
Ning H, Tao H, Weng Z, Zhao X (2016) Plasma fatty acid-binding protein 4 (FABP4) as a novel biomarker to predict gestational diabetes mellitus. Acta Diabetol 53:891–898. https://doi.org/10.1007/s00592-016-0867-8
Madhu SV, Bhardwaj S, Jhamb R, Srivastava H, Sharma S, Raizada N (2019) Prediction of gestational diabetes from first trimester serum adiponectin levels in indian women. Indian J Endocrinol Metab 23:536–539. https://doi.org/10.4103/ijem.IJEM_319_19
Sweeting AN, Wong J, Appelblom H, Ross GP, Kouru H, Williams PF et al (2019) A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn Ther 45:76–84. https://doi.org/10.1159/000486853
Kansu-Celik H, Ozgu-Erdinc AS, Kisa B, Findik RB, Yilmaz C, Tasci Y (2019) Prediction of gestational diabetes mellitus in the first trimester: comparison of maternal fetuin-A, N-terminal proatrial natriuretic peptide, high-sensitivity C-reactive protein, and fasting glucose levels. Arch Endocrinol Metab 63:121–127. https://doi.org/10.20945/2359-3997000000126
Hattan RA, Rees GK, Johnson ML (1982) Normal fetal anatomy. Radiol Clin North Am 20:271–284
Cyr DR (1983) Ultrasonic visualization of the fetal pancreas and hepatic venous circulation. Med Ultrasound 7:27–31
Hill LM, Peterson C, Rivello D, Hixson J, Belfar HL (1989) Sonographic detection of the fetal pancreas. J Clin Ultrasound 17:475–479. https://doi.org/10.1002/jcu.1870170704
Hata K, Hata T, Kitao M (1988) Ultrasonographic identification and measurement of the human fetal pancreas in utero. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 26:61–64. https://doi.org/10.1016/0020-7292(88)90197-x
Kivilevitch Z, Achiron R, Perlman S, Gilboa Y (2017) The Normal Fetal Pancreas. J Ultrasound Med Off J Am Inst Ultrasound Med 36:1997–2005. https://doi.org/10.1002/jum.14233
Akkaya H, Büke B, Uysal G (2020) Fetal pancreatic hyperechogenicity may be an early ultrasonographic sign of gestational diabetes mellitus. J Matern Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 33:2387–2394. https://doi.org/10.1080/14767058.2018.1551351
Gilboa Y, Krekora M, Perlman S, Bardin R, Kassif E, Achiron R et al (2022) Sonographic measurement of the fetal pancreas in women with gestational diabetes. Arch Med Sci 18:382–387. https://doi.org/10.5114/aoms/140578
Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJM (2018) Gestational diabetes mellitus risk score: a practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract 145:130–137. https://doi.org/10.1016/j.diabres.2018.05.001
Coustan DR, Lowe LP, Metzger BE, Dyer AR (2010) The hyperglycemia and adverse pregnancy outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol 202(654):e1-6. https://doi.org/10.1016/j.ajog.2010.04.006
Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC et al (2015) The international federation of gynecology and obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care#. Int J Gynecol Obstet 131:S173–S211. https://doi.org/10.1016/S0020-7292(15)30033-3
Chatzakis C, Cavoretto P, Sotiriadis A (2021) Gestational diabetes mellitus pharmacological prevention and treatment. Curr Pharm Des 27:3833–3840. https://doi.org/10.2174/1381612827666210125155428
Syngelaki A, Pastides A, Kotecha R, Wright A, Akolekar R, Nicolaides KH (2015) First-trimester screening for gestational diabetes mellitus based on maternal characteristics and history. Fetal Diagn Ther 38:14–21. https://doi.org/10.1159/000369970
Sirico A, Lanzone A, Mappa I, Sarno L, Słodki M, Pitocco D et al (2019) The role of first trimester fetal heart rate in the prediction of gestational diabetes: a multicenter study. Eur J Obstet Gynecol Reprod Biol 243:158–161. https://doi.org/10.1016/j.ejogrb.2019.10.019
Sirico A, Sarno L, Zullo F, Martinelli P, Maruotti GM (2019) Pregestational diabetes and fetal heart rate in the first trimester of pregnancy. Eur J Obstet Gynecol Reprod Biol 232:30–32. https://doi.org/10.1016/j.ejogrb.2018.11.003
Xi F, Chen H, Chen Q, Chen D, Chen Y, Sagnelli M et al (2021) Second-trimester and third-trimester maternal lipid profiles significantly correlated to LGA and macrosomia. Arch Gynecol Obstet 304:885–894. https://doi.org/10.1007/s00404-021-06010-0
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet (London, England) 352:837–853
Cavagnolli G, Pimentel AL, Freitas PAC, Gross JL, Camargo JL (2015) Factors affecting A1C in non-diabetic individuals: review and meta-analysis. Clin Chim Acta 445:107–114. https://doi.org/10.1016/j.cca.2015.03.024
Freitas PAC, Ehlert LR, Camargo JL (2017) Glycated albumin: a potential biomarker in diabetes. Arch Endocrinol Metab 61:296–304. https://doi.org/10.1590/2359-3997000000272
Kohzuma T, Tao X, Koga M (2021) Glycated albumin as biomarker: evidence and its outcomes. J Diabetes Complic 35:108040. https://doi.org/10.1016/j.jdiacomp.2021.108040
Zhu J, Chen Y, Li C, Tao M, Teng Y (2018) The diagnostic value of glycated albumin in gestational diabetes mellitus. J Endocrinol Invest 41:121–128. https://doi.org/10.1007/s40618-016-0605-7
Chume FC, Renz PB, Hernandez MK, Freitas PAC, Camargo JL (2021) Is there a role for glycated albumin in the diagnosis of gestational diabetes mellitus? Endocrine 72:681–687. https://doi.org/10.1007/s12020-021-02673-6
Keller SR (2004) Role of the insulin-regulated aminopeptidase IRAP in insulin action and diabetes. Biol Pharm Bull 27:761–764. https://doi.org/10.1248/bpb.27.761
Krskova K, Balazova L, Dobrocsyova V, Olszanecki R, Suski M, Chai SY et al (2020) Insulin-regulated aminopeptidase inhibition ameliorates metabolism in obese zucker rats. Front Mol Biosci 7:586225
Mostafa TM, El-Gharbawy NM, Werida RH (2021) Circulating IRAPe, Irisin, and IL-34 in relation to insulin resistance in patients with type 2 diabetes. Clin Ther 43:e230–e240. https://doi.org/10.1016/j.clinthera.2021.05.003
Tian C, Huang Z, Wen Z (2016) Associations between serum placental leucine aminopeptidase and pregnancy outcomes. Int J Gynecol Obstet 135:255–258. https://doi.org/10.1016/j.ijgo.2016.05.016
Acknowledgements
The authors would like to thank the medical staff at the Perinatology Service of our hospital for their help with the recruitment of participants and sample collection. The authors would like to thank all the participants in this study for their participation.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FYG, ABO, ITB, SYD and IY. The manuscript was written by FYG, ABO, ITB and AC. FYG, SYD, IY and AC: analyzed and interpreted of the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guleroglu, F.Y., Ozmen, A.B., Bakirci, I.T. et al. Fetal pancreas size and maternal serum biomarkers glycated albumin and insulin-regulated aminopeptidase provide no potential for early prediction of gestational diabetes mellitus. Arch Gynecol Obstet 308, 1505–1514 (2023). https://doi.org/10.1007/s00404-022-06860-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06860-2