Abstract
Purpose
Elevated levels of maternal cortisol have been hypothesized as the intermediate process between symptoms of depression and psychosocial stress during pregnancy and adverse birth outcomes. Therefore, we examined associations between cortisol levels in the second trimester of pregnancy and risks of three common birth outcomes in a nested case–control study.
Methods
This study was embedded in the PRIDE Study (n = 3,019), from which we selected all cases with preterm birth (n = 64), low birth weight (n = 49), and small-for-gestational age (SGA; n = 65), and 260 randomly selected controls, among the participants who provided a single awakening saliva sample in approximately gestational week 19 in 2012–2016. Multivariable linear and logistic regression was performed to assess the associations between continuous and categorized cortisol levels and the selected outcomes.
Results
We did not observe any associations between maternal cortisol levels and preterm birth and low birth weight. However, high cortisol levels (≥ 90th percentile) seemed to be associated with SGA (adjusted odds ratio 2.1, 95% confidence interval 0.9–4.8), in particular among girls (adjusted odds ratio 3.7, 95% confidence interval 1.1–11.9, based on eight exposed cases) in an exploratory analysis.
Conclusion
The results of this study showed no suggestions of associations between maternal awakening cortisol levels in mid-pregnancy and adverse birth outcomes, except for an increased risk of SGA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common adverse birth outcomes, including preterm birth, low birth weight, and small-for-gestational age (SGA), are associated with neonatal mortality and long-term health problems, including neurodevelopmental impairments, respiratory and gastrointestinal complications, and higher sympathetic activity, which is considered a risk factor for cardiovascular disease [1,2,3,4]. Therefore, obtaining more insight into their etiology and identifying potential measures for prevention may lead to a great impact on public health. Previous research linked maternal psychosocial problems with increased risks of these birth outcomes, but the underlying mechanisms have not been fully understood yet [5]. One of the hypothesized intermediaries for this association is maternal cortisol, a glucocorticoid [6,7,8].
Cortisol is the end metabolite of the hypothalamic–pituitary–adrenal (HPA) axis and is essential in normal brain development [9]. Throughout pregnancy, maternal cortisol levels increase twofold, and cortisol crosses the placenta, accounting for 30–40% of the variability in fetal concentrations [10]. Psychosocial stress is hypothesized to dysregulate the HPA axis through hypersecretion of cortisol, but the results of previous studies among pregnant women are conflicting in this respect [11,12,13]. Other factors, including age, medical conditions, obesity, inflammation, physical inactivity, smoking, and alcohol use may also result in elevated cortisol levels [14,15,16].
Several biological mechanisms have been proposed for linking elevated maternal cortisol levels to infant birth weight. Fetal exposure to elevated cortisol levels may dysregulate fetal autonomic nervous system activity and result in a high degree of calorie expenditure by mobilizing fetal energy stores through glycogenesis [6]. Alternatively, cortisol combined with norepinephrine may induce uterine artery vasoconstriction, resulting in reduced uterine blood flow, restricting nutrient and oxygen supply to the fetus [17]. Cortisol could also affect birth weight by stimulating the production and release of placental corticotrophin releasing hormone (CRH), leading to shortened gestation [18]. The latter may also be affected by the bi-directional association between inflammation and cortisol [19].
The results of previous studies on the associations between elevated maternal cortisol, fetal growth, and gestational age at birth were inconsistent [7, 20,21,22,23]. Study design features explain at least part of the heterogeneity in findings. These include different sampling approaches (in blood, saliva, or hair) and variations in timing of sample collection (i.e. trimester of pregnancy) and time of day at sampling. Furthermore, many previous studies had small sample sizes and limited data on covariates, possibly resulting in residual confounding.
As preterm birth, low birth weight, and SGA occur frequently, more research is needed to understand the etiology of these adverse birth outcomes and the potential role of maternal elevated cortisol levels during pregnancy therein. Using the ‘Meet in the Middle’ approach [24, 25], we hypothesized that elevated maternal salivary cortisol levels in mid-pregnancy are associated with increased risks of preterm birth, low birth weight, and SGA. A nested case–control design embedded in a large prospective cohort study was applied to examine this hypothesis while adjusting for a range of potential confounders. Furthermore, we explored whether fetal sex affects the associations between maternal salivary cortisol levels and the selected adverse birth outcomes, as the maternal HPA axis varies according to the sex of the fetus [26,27,28,29], potentially leading to higher risks among female fetuses [30].
Materials and methods
General design PRIDE Study
This study was embedded in the PRegnancy and Infant DEvelopment (PRIDE) Study [31, 32], an ongoing prospective cohort study among Dutch women enrolled in early pregnancy. In short, pregnant women of 18 years of age and above, able to read and understand the Dutch language, and not more than 16 weeks pregnant were invited to participate in the PRIDE Study by their midwife or gynecologist at their first prenatal care visit. After providing informed consent, participants completed three web-based questionnaires during pregnancy, one questionnaire two months after the estimated date of delivery, and biannual questionnaires from six months post-partum onwards. Paper-based questionnaires were available for women who could not or did not want to participate through the Internet. The baseline questionnaire was administered around gestational weeks 8–12, the second questionnaire around gestational week 17, and the third questionnaire around gestational week 34. In these questionnaires, questions were asked about demographic factors, obstetric history, maternal health, pregnancy complications, lifestyle factors, current depression or a history of depression, and environmental and occupational exposures. The first postnatal questionnaire was focused on birth outcomes and the health of the infant. Furthermore, consent was asked for review of obstetric records. The PRIDE Study was approved by the Regional Committee on Research involving Human Subjects Arnhem-Nijmegen (CMO 2009/305).
Cortisol collection and assay
Participants of the PRIDE Study were asked to optionally donate a single awakening saliva sample after completing the second prenatal questionnaire. Participants who agreed to do so received a Salivette (Sarstedt AG and Co, Nümbrecht, Germany) for saliva collection by regular mail. Samples were taken within 10 min after awakening on a working day, and before brushing teeth, eating, drinking, or smoking. The women were asked to record the date, time of awakening, and time of saliva collection. All samples were returned to the research site in a special envelope for biological materials by regular mail (median time between saliva collection and return: 3 days). The saliva samples were immediately stored at − 20 °C upon return. Samples that were received within 14 days after sampling and collected within 1 month after completing the second prenatal questionnaire were considered eligible for this study.
We used a previously described method to determine the cortisol concentration in the saliva samples selected [33]. At LDN Labor Diagnostika Nord GmbH and Co. KG, Nordhorn, Germany, the frozen samples were thawed before analysis and centrifuged for 5–10 min at 2000–3000 ×g. The concentration of cortisol in the samples was determined using the Cortisol free in Saliva ELISA Kit was used (Cortisol Saliva ELISAfree Kit). A total of 50 µL of the saliva sample, the standard reagent, and the control reagent was dispensed in microtiter wells. In addition, 50 µL of a cortisol-horseradish peroxidase conjugate for binding to the coated antibody, after which the wells were incubated at room temperature for 60 min. Subsequently, they were rinsed 3 times with 300 µL diluted wash solution and 200 µL of substrate solution was added. Again, the wells were incubated for 30 min at room temperature and 50 µL of stop solution was added to each well. The absorbance of each well was determined with a microtiter plate calibrated reader at 450 ± 10 nm within 15 min.
Nested case–control design
From the PRIDE Study participants who completed the second prenatal questionnaire between April 2012 and May 2016 and provided an eligible saliva sample, we selected all cases with preterm birth (< 37 weeks of gestation), low birth weight (< 2500 g), and/or SGA (birth weight below the 10th percentile for gestational age adjusted for parity and sex) [34]. These cases were primarily identified using validated questionnaire data [35], supplemented with data from obstetric records in case of loss to follow-up. As controls, we randomly selected subjects with saliva samples from the same time period, but without any of the above-mentioned outcomes, with a 1:4 ratio for the most prevalent outcome (i.e. SGA) for optimal study power.
Statistical analyses
Descriptive statistics were used to describe the characteristics of the women included in this nested case–control study. Using linear regression analysis, crude and adjusted β coefficients with 95% confidence intervals (CI) were estimated for the associations between maternal salivary cortisol levels and the adverse birth outcomes. These models were adjusted for potential confounders that were identified a priori by means of literature review, including maternal age (< 30, 30–34, ≥ 35 years), pre-pregnancy Body Mass Index (BMI; < 25.0 vs ≥ 25.0 kg/m2), parity (0 vs ≥ 1 previous birth), smoking during pregnancy, the presence of depressive symptoms based on the Edinburgh Depression Scale with a cut-off value of 10 [36, 37], gestational age at sampling, and days between sampling and freezing. As single salivary samples may suffer from intra-individual variability, however, we also categorized the maternal awakening cortisol levels using two different cut-off points from previous research based on cortisol levels in the control group: (1) dichotomized cortisol levels at the 75th percentile classifying women as having normal or elevated cortisol levels [33], and (2) trichotomized cortisol levels around the 50th and 90th percentile divided into low, moderate, and high [7]. Univariable logistic regression analyses were initially conducted to obtain crude odds ratios (OR) with 95% CIs for the associations between cortisol levels and the selected birth outcomes. Adjusted ORs were estimated from multivariable logistic regression models, including the same confounder set as the analyses on the continuous exposure.
In exploratory analyses, we stratified by infant sex to examine the potential effects that the sex of the infant might have on the associations between maternal cortisol levels and the selected birth outcomes. When < 5 cases were exposed, only crude ORs with Fisher exact 95% CIs were calculated using Episheet [38] instead of crude and adjusted ORs. All other statistical analyses were performed using SPSS version 25 for Windows (IBM Corp., Armonk, NY, USA).
Results
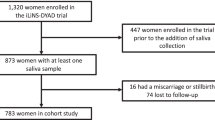
From the 3019 PRIDE Study participants who completed the second prenatal questionnaire in the study period, 1728 (57.2%) donated a saliva sample (Fig. 1). After exclusion of women who did not adhere to the sampling protocol or donated an insufficient amount of saliva, we identified 64 cases of preterm birth, 49 cases of low birth weight, and 65 cases of SGA, 42 of which were born with more than one adverse birth outcome. In addition, we sampled 260 control infants without the selected adverse birth outcomes.
The characteristics of the women and children included in the current study are presented in Table 1. Women with one of the selected birth outcomes were slightly older and were more likely to have had preeclampsia compared with control women. In addition, women with a preterm birth or an infant with low birth weight were more likely to be primiparae than control women. The latter case group was also more likely to have used alcohol during pregnancy compared with the control group. Among women in the control group, the median cortisol level was 9.0 ng/ml (interquartile range 6.5–12.3), with the 90th percentile at 14.8 ng/ml. The median cortisol levels in the case groups were in the same order of magnitude.
After correction for covariates, continuous cortisol levels were not associated with preterm birth (β = 0.40, 95% CI − 0.98 to 1.78), low birth weight (β = 0.64, 95% CI − 0.90 to 2.17), and SGA (β = 0.45, 95% CI − 0.92 to 1.81; Table 2). Restricting the analyses to cases with a single outcome moved the adjusted βs for preterm birth and SGA closer to the null value, whereas the adjusted β for low birth weight increased. However, this observation was based on only three cases.
In Table 3, the crude and adjusted ORs with their 95% CIs are shown for the associations between the categorized cortisol levels and the selected birth outcomes. We did not observe associations between maternal awakening salivary cortisol levels and preterm birth and low birth weight. A high cortisol level (i.e. ≥ 90th percentile), however, seemed to be associated with an increased risk of SGA (adjusted OR 2.1; 95% CI 0.9–4.8). Restricting the analyses to cases with a single outcome yielded comparable risk estimates, with slightly higher point estimates for SGA (Table 4).
After stratification by infant sex in the exploratory analyses, we did not observe associations between continuous maternal cortisol levels and preterm birth (boys: adjusted β = -0.48 [95% CI − 2.32 to 1.36], girls: adjusted β = 0.73 [95% CI − 1.36 to 2.83]), low birth weight (boys: adjusted β = 0.83 [95% CI − 1.21 to 2.88], girls: adjusted β = 1.00 [95% CI − 1.20 to 3.20]), and SGA (boys: adjusted β = 0.08 [95% CI − 1.60 to 1.76], girls: adjusted β = 1.31 [95% CI − 0.80 to 3.43]). The results of the stratified analyses on the categorical exposure variables are shown in Table 5. Among women who delivered a girl, a high cortisol level was associated with preterm birth (adjusted OR 4.3; 95% CI 1.1–16.9) and SGA (adjusted OR 3.7; 95% CI 1.1–11.9). We did not observe any associations among women who delivered a boy.
Discussion
In this study, we did not identify strong associations between mid-pregnancy maternal awakening salivary cortisol levels and the occurrence of preterm birth and low birth weight. However, a possible association was observed between high cortisol levels in mid-pregnancy (i.e. ≥ 90th percentile) and SGA. In the exploratory analyses, this risk of SGA was only increased among female infants, just as the risk of preterm birth, but these analyses relied on small sample sizes, resulting in unstable effect estimates.
Although cortisol has repeatedly been hypothesized as one of the biological intermediates linking prenatal psychosocial stress to adverse birth outcomes [6,7,8], studies examining the associations between maternal cortisol levels during pregnancy and adverse birth outcomes showed inconsistent results. Reasons for these inconsistencies include differences in biomarkers for cortisol, the timing and extent of biomarker collection throughout the day, and the variability in the timing of assessment during gestation, measuring in early, mid or late pregnancy [39, 40]. Some, mostly small, studies found associations between elevated maternal cortisol levels and the risk of preterm birth in the entire population or in subgroups [19, 41, 42], although publication bias may be an issue here. Other published studies did not observe associations between maternal cortisol levels during pregnancy and preterm birth [20, 43, 44], in line with our results. In a recent meta-analysis among 1606 maternal–fetal dyads [21], a negative association was observed between maternal salivary cortisol and infant birth weight. However, the risk of low birth weight, a clinically relevant outcome measure, was not assessed. In a large prospective cohort study of 2810 women not included in this meta-analysis, no associations were observed between elevated serum cortisol levels measured in early pregnancy and offspring birth weight [7]. Furthermore, cortisol was not associated with self-reported measures of psychological functioning among pregnant women in several studies [11,12,13, 23], making it unlikely that cortisol can be classified as biomarker of the mechanistic pathway linking maternal psychosocial problems to preterm birth and birth weight according to the ‘Meet in the Middle’ approach. Alternative markers of stress related to the autonomous nervous system and inflammatory response system may also play a more prominent role in the association between maternal prenatal stress and these birth outcomes [28, 45], and should be taken into account in future studies.
In the same prospective cohort study of 2810 women, an association was observed between high morning cortisol levels (≥ 90th percentile) and an increased risk of SGA, concordant with our findings. Several potential pathways underlying the associations between elevated maternal cortisol levels and adverse birth outcomes have been suggested, including dysregulation of the fetal autonomic nervous system [6], vasoconstriction of the uterine artery resulting in reduced uterine blood flow [17], and stimulation of the production and release of placental CRH [18]. Specifically for SGA, an association with disturbed expression and/or activity of placental 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), an enzyme that protects the fetus from high levels of maternal cortisol, has been observed [46,47,48,49,50]. Interestingly, decreased functioning of 11β-HSD2 in SGA infants has only been observed in pregnancies with female fetuses [51]. Recent findings on sex-specific differences in systemic glucocorticoid imbalance [52] also strengthen the plausibility of our finding that elevated maternal salivary cortisol increases the risk of SGA among female fetuses only. Likewise, previous research indicated that the effects of depression during pregnancy could have a different impact on the two sexes [53].
Strengths of this study include the nested case–control design with embedding in a large prospective cohort study. Due to this design, we were able to include a relatively large number of cases compared to previous smaller studies [6, 23, 41]. This made it possible to perform stratified analyses for infant sex, which could modify associations between increased cortisol levels and adverse birth outcomes. The design also led to the availability of prospectively collected information on many maternal characteristics, which were used to adjust for potential confounding effects on the associations of interest. Previous studies showed that maternal characteristics, such as parity, smoking behavior, and BMI, could have an influence on maternal cortisol levels [7, 54, 55]. Adjustment for these factors was often not completely or not at all possible in previous studies, resulting in potentially biased effect estimates due to residual confounding.
A limitation of the current study is the collection of only a single awakening saliva sample to determine the cortisol level, whereas multiple measurements during the day or on consecutive days might be preferred. In a previous study validating this approach, however, we showed that a single awakening salivary sample could reliably distinguish between women having normal and elevated cortisol levels [33]. Nevertheless, we were unable to assess the cortisol awakening response and exposure patterns or trajectories throughout pregnancy with a single sample. As the saliva sample was collected in mid-pregnancy, we could not examine the associations between adverse birth outcomes and fetal exposure to elevated cortisol levels in early or late pregnancy. The critical exposure windows for the risks of preterm birth, low birth weight, and SGA are currently unknown [7, 56], but most previous studies collected the biomarkers for cortisol around gestational week 20, comparable to our study. By performing stratified exploratory analyses, we obtained more insight into the potential sex-dependent effects of elevated maternal cortisol levels. Due to small numbers of exposed cases in some of these exploratory analyses, however, we cannot draw firm conclusions on the sex-specific associations. Therefore, these analyses should be repeated in larger studies.
Conclusion
The results of this nested case–control study showed no indications that maternal cortisol levels in mid-pregnancy are strongly associated with increased risks of preterm birth and low birth weight, but they may be associated with an increased risk of SGA. Infant sex seemed to influence this association. These findings help to understand the etiology and the onset of the occurrence of adverse birth outcomes and contribute to growing knowledge of the complex biological pathways linking maternal psychosocial problems to adverse birth outcomes.
References
Wang ML, Dorer DJ, Fleming MP, Catlin EA (2004) Clinical outcomes of near-term infants. Pediatrics 114:372–376. https://doi.org/10.1542/peds.114.2.372
Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371:75–84. https://doi.org/10.1016/s0140-6736(08)60074-4
Saigal S, Doyle LW (2008) An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371:261–269. https://doi.org/10.1016/s0140-6736(08)60136-1
Van Deutekom AW, Chinapaw MJM, Gademan MGJ, Twisk JWR, Gemke RJBJ, Vrijkotte TGM (2016) The association of birth weight and infant growth with childhood autonomic nervous system activity and its mediating effects on energy-balance-related behaviours: the ABCD Study. Int J Epidemiol 45:1079–1090. https://doi.org/10.1093/ije/dyw236
Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis C-L, Koren G, Steiner M, Mousmanis P, Cheung A, Radford K, Martinovic J, Ross LE (2013) The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 74:e321–e341. https://doi.org/10.4088/jcp.12r07968
Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH (2009) Prenatal depression restricts fetal growth. Early Hum Dev 85:65–70. https://doi.org/10.1016/j.earlhumdev.2008.07.002
Goedhart G, Vrijkotte TGM, Roseboom TJ, van der Wal MF, Cuijpers P, Bonsel GJ (2010) Maternal cortisol and offspring birthweight: results from a large prospective cohort study. Psychoneuroendocrinology 35:644–652. https://doi.org/10.1016/j.psyneuen.2009.10.003
Valsamakis G, Papatheodorou D, Chalarakis N, Manolikaki M, Margeli A, Papassotiriou I, Barber TM, Kumar S, Kalantaridou S, Mastorakos G (2020) Maternal chronic stress correlates with serum levels of cortisol, glucose and C-peptide in the fetus, and maternal non chronic stress with fetal growth. Psychoneuroendocrinology 114:104591. https://doi.org/10.1016/j.psyneuen.2020.104591
Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG (2006) Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol 572:31–44. https://doi.org/10.1113/jphysiol.2006.105254
Gitau R, Cameron A, Fisk NM, Glover V (1998) Fetal exposure to maternal cortisol. Lancet 352:707–708. https://doi.org/10.1016/s0140-6736(05)60824-0
Petraglia F, Hatch MC, Lapinski R, Stomati M, Reis FM, Cobellis L, Berkowitz GS (2001) Lack of effect of psychosocial stress on maternal corticotropin-releasing factor and catecholamine levels at 28 weeks’ gestation. J Soc Gynecol Investig 8:83–88
Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM (2009) Stress questionnaires and stress biomarkers during pregnancy. J Womens Health 18:1425–1433. https://doi.org/10.1089/jwh.2008.1102
Vlenterie R, Geuijen PM, van Gelder MMHJ, Roeleveld N (2021) Questionnaires and salivary cortisol to measure stress and depression in mid-pregnancy. PLoS ONE 16:e0250459. https://doi.org/10.1371/journal.pone.0250459
Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder T (2014) Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology 39:132–140. https://doi.org/10.1016/j.psyneuen.2013.10.007
Melin EO, Thunander M, Landin-Olsson M, Hillman M, Thulesius HO (2014) Depression, smoking, physical inactivity and season independently associated with midnight salivary cortisol in type 1 diabetes. BMC Endocr Disord 14:75. https://doi.org/10.1186/1472-6823-14-75
Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ (2015) Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology 62:301–318. https://doi.org/10.1016/j.psyneuen.2015.08.014
Field T, Diego M (2008) Cortisol: the culprit prenatal stress variable. Int J Neurosci 18:1181. https://doi.org/10.1080/00207450701820944
Sandman CA, Glynn L, Dunkel Schetter C, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C (2006) Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides 27:1457–1463. https://doi.org/10.1016/j.peptides.2005.10.002
Bandoli G, Jelliffe-Pawlowski LL, Feuer SK, Liang L, Oltman SP, Paynter R, Ross KM, Dunkel Schetter C, Ryckman KK, Chambers CD (2018) Second trimester serum cortisol and preterm birth: an analysis by timing and subtype. J Perinatol 38:973–981. https://doi.org/10.1038/s41372-018-0128-5
Stewart CP, Oaks BM, Laugero KD, Ashorn U, Harjunmaa U, Kumwenda C, Chaima D, Maleta K, Ashorn P, Dewey KG (2015) Maternal cortisol and stress are associated with birth outcomes, but are not affected by lipid-based nutrient supplements during pregnancy: an analysis of data from a randomized controlled trial in rural Malawi. BMC Pregnancy Childbirth 15:346. https://doi.org/10.1186/s12884-015-0793-8
Cherak SJ, Giesbrecht GF, Metcalfe A, Ronksley PE, Malebranche ME (2018) The effect of gestational period on the association between maternal prenatal salivary cortisol and birth weight: a systematic review and meta-analysis. Psychoneuroendocrinology 94:49–62. https://doi.org/10.1016/j.psyneuen.2018.04.023
Gilles M, Otto H, Wolf IAC, Scharnholz B, Peus V, Schredl M, Sütterlin MW, Witt SH, Rietschel M, Laucht M, Deuschle M (2018) Maternal hypothalamus-pituitary-adrenal (HPA) system activity and stress during pregnancy: effects on gestational age and infant’s anthropometric measures at birth. Psychoneuroendocrinology 94:152–161. https://doi.org/10.1016/j.psyneuen.2018.04.022
Smew AI, Hedman AM, Chiesa F, Ullemar V, Andolf E, Pershagen G, Almqvist C (2018) Limited association between markers of stress during pregnancy and fetal growth in ‘Born into Life’, a new prospective birth cohort. Acta Paediatr 107:1003–1010. https://doi.org/10.1111/apa.14246
Vineis P, Perera F (2007) Molecular epidemiology and biomarkers in etiologic cancer research: the new in light of the old. Cancer Epidemiol Biomark Prev 16:1954–1965. https://doi.org/10.1158/1055-9965.epi-07-0457
Demetriou CA, van Veldhoven K, Relton C, Stringhini S, Kyriacou K, Vineis P (2015) Biological embedding of early-life exposures and disease risk in humans: a role for DNA methylation. Eur J Clin Invest 45:303–332. https://doi.org/10.1111/eci.12406
DiPietro JA, Costigan KA, Kivlighan KT, Chen P, Laudenslager ML (2011) Maternal salivary cortisol differs by fetal sex during the second half of pregnancy. Psychoneuroendocrinology 36:588–591. https://doi.org/10.1016/j.psyneuen.2010.09.005
Appleton AA, Armstrong DA, Lesseur C, Lee J, Padbury JF, Lester BM, Marsit CJ (2013) Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity. PLoS ONE 8:e74691. https://doi.org/10.1371/journal.pone.0074691
Giesbrecht GF, Campbell T, Letourneau N, APrON Study Team (2015) Sexually dimorphic adaptations in basal maternal stress physiology during pregnancy and implications for fetal development. Psychoneuroendocrinology 56:168–178. https://doi.org/10.1016/j.psyneuen.2015.03.013
Braithwaite EC, Hill J, Pickles A, Glover V, O’Donnell K, Sharp H (2018) Associations between maternal prenatal cortisol and fetal growth are specific to infant sex: findings from the wirral child health and development study. J Dev Orig Health Dis 9:425–431. https://doi.org/10.1017/s2040174418000181
Carpenter T, Grecian SM, Reynolds RM (2017) Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis 8:244–255. https://doi.org/10.1017/s204017441600074x
Van Gelder MMHJ, Bretveld RW, Roukema J, Steenhoek M, van Drongelen J, Spaanderman MEA, van Rumpt D, Zielhuis GA, Verhaak CM, Roeleveld N (2013) Rationale and design of the PRegnancy and infant DEvelopment (PRIDE) Study. Paediatr Perinat Epidemiol 27:34–43. https://doi.org/10.1111/ppe.12023
Van Gelder MMHJ, Merkus PJFM, van Drongelen J, Swarts JW, van de Belt TH, Roeleveld N (2020) The PRIDE study: evaluation of online methods of data collection. Paediatr Perinat Epidemiol 34:484–494. https://doi.org/10.1111/ppe.12618
Vlenterie R, Roeleveld N, van Gelder MMHJ (2016) Single awakening salivary measurements provide reliable estimates of morning cortisol levels in pregnant women. Psychoneuroendocrinology 74:295–301. https://doi.org/10.1016/j.psyneuen.2016.09.009
Visser GHA, Eilers PHC, Elferink-Stinkens PM, Merkus HMWM, Wit JM (2009) New dutch reference curves for birthweight by gestational age. Early Hum Dev 85:737–744. https://doi.org/10.1016/j.earlhumdev.2009.09.008
Van Gelder MMHJ, Vorstenbosch S, Derks L, te Winkel B, van Puijenbroek EP, Roeleveld N (2017) Web-based questionnaires to assess perinatal outcome proved to be valid. J Clin Epidemiol 90:136–143. https://doi.org/10.1016/j.jclinepi.2017.07.004
Pop VJ, Komproe IH, van Son MJ (1992) Characteristics of the edinburgh post natal depression scale in the Netherlands. J Affect Disord 26:105–110. https://doi.org/10.1016/0165-0327(92)90041-4
Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen BR, van Baar A, Pop V (2011) Validation of the edinburgh depression scale during pregnancy. J Psychosom Res 70:385–389. https://doi.org/10.1016/j.jpsychores.2010.07.008
Rothman K (2013) Episheet: spreadsheets for the analysis of epidemiologic data. http://www.epidemiolog.net/studymat/. Accessed 17 Jan 2022
Jones NM, Holzman CB, Zanella AJ, Leece CM, Rahbar MH (2006) Assessing mid-trimester salivary cortisol levels across three consecutive days in pregnant women using an at-home collection protocol. Paediatr Perinat Epidemiol 20:425–437. https://doi.org/10.1111/j.1365-3016.2006.00744.x
Skoluda N, Linnemann A, Nater UM (2016) The role of week (end)-day and awakening time on cortisol and alpha-amylase awakening responses. Stress 19:333–338. https://doi.org/10.1080/10253890.2016.1174850
Field T, Henandez-Reif M, Diego M, Figueiredo B, Schanberg S, Kuhn C (2006) Prenatal cortisol, prematurity and low birthweight. Infant Behav Dev 29:268–275. https://doi.org/10.1016/j.infbeh.2005.12.010
Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, Ross RG (2016) Measures of maternal stress and mood in relation to preterm birth. Obstet Gynecol 127:545–552. https://doi.org/10.1097/aog.0000000000001287
Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Fong Chen M, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW (2009) Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol 169:1319–1326. https://doi.org/10.1093/aje/kwp061
Kramer MS, Lydon J, Goulet L, Kahn S, Dahhou M, Platt RW, Sharma S, Meaney MJ, Séquin L (2013) Maternal stress/distress, hormonal pathways and spontaneous preterm birth. Paediatr Perinat Epidemiol 27:237–246. https://doi.org/10.1111/ppe.12042
Nazzari S, Fearon P, Rice F, Dottori N, Ciceri F, Molteni M, Frigerio A (2019) Beyond the HPA-axis: exploring maternal prenatal influences on birth outcomes and stress reactivity. Psychoneuroendocrinology 101:253–262. https://doi.org/10.1016/j.psyneuen.2018.11.018
Kajantie E, Dunkel L, Turpeinen U, Stenman U-H, Wood PJ, Nuutila M, Andersson S (2003) Placental 11 beta-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab 88:493–500. https://doi.org/10.1210/jc.2002-021378
Dy J, Guan H, Sampath-Kumar R, Richardson BS, Yang K (2008) Placental 11beta-hydroxysteroid dehydrogenase type 2 in pregnancies complicated with idiopathic intrauterine growth restriction: evidence that this is associated with an attenuated ratio of cortison to cortisol in the umbilical artery. Placenta 29:193–200. https://doi.org/10.1016/j.placenta.2007.10.010
Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H, Surbek D, Hocher B, Mohaupt MG (2009) Prematurity is related to high placental cortisol in preeclampsia. Pediatr Res 65:198–202. https://doi.org/10.1203/pdr.0b013e31818d6c24
Börzsönyi B, Demendi C, Pajor A, Rigó J Jr, Marosi K, Agota A, Nagy ZB, Gábor Joó J (2012) Gene expression patterns of the 11β-hydroxysteroid dehydrogenase 2 enzyme in human placenta from intrauterine growth restriction: the role of impaired feto-maternal glucocorticoid metabolism. Eur J Obstet Reprod Biol 161:12–17. https://doi.org/10.1016/j.ejogrb.2011.12.013
Zhu Z, Liu Q (2015) Relationship between 11β-HSD2 mRNA and insulin sensitivity in term small-for-gestational age neonates after birth. Int J Clin Exp Pathol 8:928–932
Mericq V, Medina P, Kakarieka E, Márquez L, Johnson MC, Iñiguez G (2009) Differences in expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 and 2 in human placentas in term pregnancies according to birth weight and gender. Eur J Endocrinol 161:419–425. https://doi.org/10.1530/eje-09-0308
Siemiątkowska A, Kosicka K, Szpera-Goździewicz A, Krzyścin M, Bręborowicz GH, Główka FK (2019) Cortisol metabolism in pregnancies with small for gestational age neonates. Sci Rep 9:17890. https://doi.org/10.1038/s41598-019-54362-0
Edwards RC, Hans SL (2016) Prenatal depressive symptoms and toddler behavior problems: the role of maternal sensitivity and child sex. Child Psychiatry Hum Dev 47:696–707. https://doi.org/10.1007/s10578-015-0603-6
Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C (2001) Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE family study. Eur J Endocrinol 145:1–9. https://doi.org/10.1530/eje.0.1450001
Steptoe A, Ussher M (2006) Smoking, cortisol and nicotine. Int J Psychophysiol 59:228–235. https://doi.org/10.1016/j.ijpsycho.2005.10.011
Entringer S, Wadhwa PD, Buss C, Lu MC (2011) The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol 38:351–384. https://doi.org/10.1016/j.clp.2011.06.007
Funding
This work was supported by the Netherlands Organisation for Health Research and Development (ZonMw; Grant number 836011020).
Author information
Authors and Affiliations
Contributions
RV: protocol development, data collection, data analysis, manuscript writing. JBP: protocol development, manuscript editing. NR: project development, manuscript editing. MMHJvG: project development, data management, data analysis, manuscript writing. All authors contributed to the study conception and design. Data collection and statistical analyses were performed by RV and MvG. The first draft of the manuscript was written by RV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Regional Committee on Research involving Human Subjects Arnhem-Nijmegen (October 5, 2010; CMO 2009/305).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vlenterie, R., Prins, J.B., Roeleveld, N. et al. Associations between maternal awakening salivary cortisol levels in mid-pregnancy and adverse birth outcomes. Arch Gynecol Obstet 306, 1989–1999 (2022). https://doi.org/10.1007/s00404-022-06513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06513-4