Abstract
Objectives
To analyse prenatal parameters predicting biventricular (BV) outcome in pulmonary atresia with intact ventricular septum/critical pulmonary stenosis (PAIVS/CPS).
Methods
We evaluated 82 foetuses from 01/08 to 10/18 in 3 centres in intervals 1 (< 24 weeks), 2 (24–30 weeks) and 3 (> 30 weeks).
Results
61/82 (74.4%) were livebirths, 5 (8.2%) lost for follow-up, 3 (4.9%) had compassionate care leaving 53 (64.6% of the whole cohort and 86.9% of livebirths) with intention to treat. 9 died, 44/53 (83.0%) survived. 24/38 (63.2%) with information on postnatal outcome had BV outcome, 14 (36.8%) non-BV outcome (2 × 1.5 circulation). One with BV outcome had prenatal valvuloplasty. Best single parameter for BV outcome was tricuspid/mitral valve (TV/MV) ratio (AUC 0.93) in intervals 2 and 3 (AUC 0.92). Ventriculo-coronary-arterial communications (VCAC) were present in 11 (78.6%) in non-BV outcome group vs. 2 (8.3%) in BV outcome group (p < 0.001). Tricuspid insufficiency (TI)-Vmax > 2.5 m/s was present in BV outcome group in75.0% (18/24) vs. 14.3% (2/14) in non-BV outcome group. Including the most predictive markers (VCAC presence, TI- Vmax < 2.5 m/s, TV/MV ratio < cutoff) to a score, non-BV outcome was correctly predicted when > 1 criterion was fulfilled in all cases. After recently published criteria for foetal intervention, only 4/9 (44.4%) and 5/14 (35.7%) in our interval 2 + 3 with predicted non-BV outcome would have been candidates for intervention. Two (1 × intrauterine intervention) in interval 2, two in interval 3 reached BV outcome and one 1.5 circulation without intervention.
Conclusion
TV/MV ratio as simple parameter has high predictive value. After our score, non-BV outcome was correctly predicted in all cases. Criteria for foetal intervention must further be evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary atresia with intact ventricular septum (PAIVS) is a rare, heterogenous cardiac anomaly with an incidence of 4–5/100,000 live births [1]. Outcomes have improved over time, but are still guarded with reported one- and 5-year survival rates of 70–75% and 63–67%, depending on the type of postnatal circulation [2, 3]
While in Germany prenatal detection rate for congenital heart defect (CHD) is low with < 20% for all CHD and about 42% for severe CHD [4], in UK two-thirds of cases with PAIVS/critical pulmonary stenosis (CPS) are detected prenatally [5]. The rate of termination of pregnancy (TOP) in PAIVS/CPS is up to 60%, especially when univentricular (UV) outcome is supposed [1]. Therefore, prediction of circulation outcome is an important aspect for parental counselling. Prenatal parameters have been investigated in smaller cohorts and there is ongoing debate on best predictors of postnatal circulation [5,6,7,8,9,10,11,12,13]. Foetal pulmonary valvuloplasty is performed in a few centres to obviate the consequences of postnatal non-biventricular (BV) circulation; however, treated cases are few, outcomes are limited and best selection criteria undetermined yet [14].
The aim of our study was to describe outcomes in prenatally diagnosed PAIVS/CPS, to analyse foetal echocardiographic parameters for predicting BV outcome and growth rate of these during gestation in the largest cohort to date.
Materials and methods
The initial database found 94 foetuses with a prenatal diagnosis of PAIVS/CPS between 01/2008 and 10/20,182,017 in three centres, division of prenatal medicine Justus-Liebig-University Giessen Germany, Praenatal plus in Cologne Germany and Fetal Cardiology Unit in Ukrainian Children's Cardiac Center in Kiew Ukraine.
Informed parental consent to anonymised analysis of the data was given and the study was approved by the local research ethics committee of the faculty of medicine of Justus-Liebig university in Giessen (protocol number 219/16).
Nine patients with additional intracardiac abnormalities, e.g. Ebstein‘s anomaly or cases dominated by TV dysplasia were excluded as well as two cases with twin–twin transfusion syndrome (TTTS) and one with Williams–Beuren syndrome (WBS) as the mechanism of PS is usually different in those cases.
The final cohort comprised 82 foetuses. Foetal echocardiography was performed according to guidelines of ISUOG by a segmental approach and defined anatomical planes with colour pulsed-wave Doppler [15]. 5 MHz, 7.5 MHz or 9 MHz sector or curved array probes were used (Toshiba Aplio 500, Toshiba Aplio XG, Toshiba Medical, Neuss, Germany, Philips EPIQ 7, Philips iU22, Canon Aplio i900,GE Voluson E10).Fetal karyotyping was offered and included chromosome analysis and fluorescent in situ hybridization for microdeletion 22q11.2. Parental counselling by pediatric cardiologists was part of the prenatal work-up. Data were collected from medical files, ultrasound images and videos, whenever available. A complete foetal echocardiography was assessed. In contrast to PAIVS, CPS was diagnosed when a pinhole jet flow through the pulmonary valve (PV) was present. In all cases, a holosystolic reversal of flow within the arterial duct was diagnosed. Presence of ventriculo-coronary-arterial communications (VCAC), was documented.
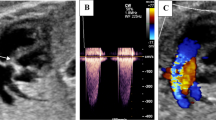
An example of prenatal PAIVS is shown in Fig. 1.
Tricuspid valve (TV) and mitral valve (MV) were measured at diastole when the diameter reached maximum before closing of atrioventricular valves, aortic and pulmonary valve at end-systole (Figs. 2, 3).
Most measurements were done prospectively. Missing parameters were retrieved from stored images/videos by repeated measurements and mean value was determined. Investigators were blinded to outcome. Right and left ventricular lengths were measured from the centre of atrioventricular valves to the endocardial surface in end-diastole. Z-scores were generated if required, using algorithm of Schneider et al. 2005 [16]. Continuous-wave Doppler of TI was recorded to measure peak velocity (Vmax). For data analysis, R version 3.5.2 was used.
Continuous variables are reported as mean ± SD or median (range) depending on the data distribution. Categorial data are expressed as frequencies and percentages (%) and compared among groups using Fisher’s exact test, t-test for normally distributed data or Mann–Whitney U test for not normally distributed data in interval-scaled variables. Normal distribution was tested using Shapiro test. Number of examinations and gestation week varied widely between the foetuses from different centres; therefore, data analysis was challenging. We used a model similar to that described by Gardiner and evaluated the measurements at three gestation intervals: interval 1 < 24 weeks, interval 2 from 24–30 weeks to and interval 3 > 30 weeks [5]. Mean for all z-scores and ratios was calculated for each foetus and interval. For echocardiographic parameters, Receiver-Operating-Characteristics (ROC) curves were calculated for establishing cutoff values. We included the most predictive parameters, TV/MV ratio, TI-Vmax < 2.5 m/s and presence of VCAC into a scoring system for non-BV outcome.
Longitudinal assessment of growth rate of pulmonary valve (PV), TV and RV lengths during gestation was calculated by measuring the change in dimension between the first and last fetal examination and dividing the result by number of weeks between both examinations [17].
Results
Description of prenatal cohort
Eighty-two pregnancies with prenatal diagnosis of PAIVS/CPS in three different centres were retrieved. There were seventy-nine (96.3%) singletons and three (3.7%) twins. Median maternal age was 29 (18–42) years. The rate for assisted reproduction was three of 82 (3.7%). Sixty-three foetuses presented with PAIVS (76.8%), nineteen (23.2%) had CPS.
Diagnosis of CHD was made in median 22.2 (12–31) weeks of gestation.
In one CPS, progression to PAIVS during pregnancy was observed. One patient had prenatal valvuloplasty in 25 + 5 weeks.
Cardiac, extracardiac and genetic anomalies
Thirty-four (41.5%) foetuses had VCAC. Fifty-eight (71.7%) presented with TI. EFE within the right ventricle was present in twenty-five foetuses (30.5%) and restriction of the FO in six (7.3%) cases (Table 1). In five cases, VCAC was only diagnosed postnatally and three cases of VCAC were presumed prenatally, but not confirmed postnatally.
In seventeen (20.7%), foetuses karyotype was normal. We encountered one postnatally diagnosed trisomy 21.
In three (3.7%) patients, extracardiac anomalies were detected. One presented with ureteral stenosis and hypospadias; two had a single umbilical artery.
Outcome
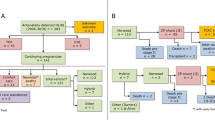
In eighteen of 82 (22.0%) cases, parents opted for TOP and two foetuses (2.4%) were lost for follow-up during prenatal period. One early IUD (1.2%) in 14 weeks occurred in PAIVS with small RV, absent A-wave in ductus venosus and increased nuchal translucency. Karyotype was normal; array diagnostic was refused. Sixty-one (74.4%) were livebirths, five (8.2%) were lost for follow-up postnatally, in three cases (4.9%) parents decided for compassionate care, leaving fifty-three (64.6% of the whole cohort and 86.9% of livebirths) with intention-to-treat. Nine (17.0%) of them died during follow-up; accordingly, intention-to-treat survival rate was forty-four of 53 (83.0%).
All demises within intention-to-treat group occured within nine months, seven of 9 (77.8%) died during the neonatal period. Two of them had non-BV outcome; in the other cases, circulation outcome was undetermined (Table 2). Median follow-up for surviving patients was 22.5 months.
In thirty-eight of 53 (71.7%) patients, information regarding BV/non-BV including 1.5 circulation was available.
Seven of them were patients with early neonatal death and in eight patients, outcome was still undetermined, mainly due to short follow-up at the time of evaluation.
Twenty-four of 38 (63.2%) patients had BV outcome, fourteen of 38 (36.8%) had non-BV outcome, two (1.4%) within this subgroup had 1.5 circulation. (Fig. 4).
Concerning perinatal data, delivery was at 38.43 (31.7–41.9) weeks. Nine (14.8%) were preterm deliveries including four within 32–34 weeks. A caesarean section was performed in 70%, most of them (66.7%) were primary caesarean sections. The main reasons for primary caesarean section were practical reasons as desire for planned delivery in case of remote home from the heart centre.
Median percentile of birth weight was 27.5. Perinatal data were comparable in both outcome groups.
Regarding intracardiac anomalies in BV versus non-BV outcome, there were significant more cases with VCAC within the non-BV group (11/14 (78.6%) versus 2/24 (8.3%) in BV outcome group) and more cases with TI-Vmax > 2.5 m/s in BV outcome group (18/24 (75.0%) versus 2/14 (14.3%) in non-BV outcome group). Two patients with VCAC had BV outcome, another patient with VCAC had 1.5 circulation (was documented in the non-BV outcome group). The rate for endocardial fibroelastosis (EFE) was not significantly different.
Evaluation of parameters predicting BV versus non-BV outcome
We evaluated z-scores of PV, TV, pulmonary arteries, TV/MV ratio, PV/AV ratio, RV/LV length ratios and TI-Vmax in interval 1 (< 24 weeks), interval 2 (24–30 weeks) and interval 3 > 30 weeks.
In all intervals, ratios of TV/MV and PV/AV performed better in predicting circulatory outcome than zTV or zPV alone.
The best predicting parameter for BV versus non-BV outcome in interval 1 was PV/AV ratio (area under the curve (AUC 0.82) followed by TV/MV ratio (AUC 0.78)). Measurements in interval I were limited, because patients often were referred later.
In interval 2, the best predicting parameter was TV/MV ratio and RV/LV length ratio (both AUC 0.93). Cutoff for TV/MV ratio was 0.62 and Cutoff for RV/LV length ratio was 0.70 in this interval.
In interval 3, measurements were available in most patients. The best predicting parameter as well as in interval 2 was TV/MV ratio (AUC 0.92, cutoff 0.71), the second best was RV/LV length ratio (AUC 0.90, cutoff 0.68).
Available measurement, AUC values, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and cutoffs within the three intervals are summarised in Table 3. ROC curves for the best predictive parameter, TV/MV ratio, for intervals 2 and 3 with relevant number of measurements are shown in Figs. 5 and 6.
One patient had prenatal pulmonary valve valvuloplasty in 25 + 5 weeks in our centre and BV outcome was achieved. The patient had absence of VCAC, TI-Vmax > 2.5 m/s. Mean zTV was − 4.42, TV/MV ratio was 0.53 and RV/LV length ratio was 0.60 in mean before intervention; so non-BV outcome would have been predicted after TV/MV ratio and RV/LV length ratio cutoff in our evaluation for this interval.
As described above, BV outcome was associated with TI-Vmax > 2.5 m/s and non-BV outcome with the presence of VCAC, so we combined these parameters with TV/MV ratio as best single parameter to a score. Non-BV outcome was correctly predicted in all cases if more than one criteria were fulfilled. False-positive rate for the prediction score was 17%.
AUC values, sensitivity, specificity, PPV, NPV for the score in the 3 different gestation intervals are summarised in Table 3. ROC curves of each predictive parameter, TV/MV ratio, TI-Vmax < 2.5 m/s and presence of VCAC for interval 2 with the biggest clinical impact are shown in Fig. 7.
Longitudinal assessment of growth of PV, TV and RV lengths during gestation
There were no significantly different growth rates in patients with BV outcome and non-BV outcome for all three parameters. Only for TV, a tendency towards lower growth rates for patients with non-BV outcome (0.18 mm/week in non-BV outcome group versus 0.29 mm/week in BV outcome group, p value = 0.08) was seen.
Discussion
The aim of the study was to describe survival rate and, second, to predict BV outcome by prenatal echocardiographic parameters.
Intention-to-treat survival rate (median follow-up 22.5 months) was 44/53 (83.0%) which fits to reported one-, 5- and 10-year survival rates ranging from 63 to 97% [2, 3, 18, 19]. Most deaths were neonatal deaths which is in line with others [18].
In our cohort, the best single, echocardiographic parameter to predict BV outcome was TV/MV ratio > 24 weeks. We included TV/MV ratio as best single parameter in combination with presence of VCAC and TI-Vmax < 2.5 m/s to a score. Non-BV outcome was correctly predicted in all our cases if more than one criteria were fulfilled. False-positive rate of the score is 17%, respectively, which is similar to previous data and should be considered for parental counselling [10].
In our cohort, almost two-thirds (63.2%) with evaluable circulation outcome had BV outcome, similar to published data [10, 20, 21]. TOP rate was high (22.0%). There exist reported TOP rates of up to 60%, and more parents will obviously opt for TOP when UV outcome is predicted. [1]
Data for prenatal predictors in PAIVS/CPS are limited with small cohorts of 15–34 analyzable cases [5,6,7,8, 10, 12, 20, 21] To our knowledge, this is one of the largest cohorts in literature, with still small dataset of measurements, especially < 24 weeks.
Our results for TV/MV ratio showed AUC of 0.93 (cutoff 0.62) for 24–30 weeks and AUC of 0.92 (cutoff 0.71) > 30 weeks.
This is in line with Lowenthal et al. who find TV/MV ratio > 0.63 as strong predictor of favourable postnatal TV z-score in CPS/PAIVS [20]. Others also incorporated TV/MV ratio into their predictive scores [5, 10, 21]. Gardiner et al. developed scores for different gestation intervals including TV and PV z-scores and TV/MV ratio; they found a combination of TV/MV ratio with zTV as best predictor > 31 weeks [5]. Roman et al. developed a score by combining TV/MV ratio < 0.7, RV/LV length < 0.6, TV inflow duration > 31.5% and presence of sinusoids. When three criteria are fulfilled, non-BV outcome is predicted with sensitivity of 100%, specificity of 75%, PPV of 88% and NPV 100%. [10]. Recently, Gottschalk et al. reported a scoring system including (TR) < 2 m/s, right ventricle/left ventricle length ratio ≤ 0.6, and presence of VCAC with high sensitivity and specificity of 100% for prediction of UV circulation [12].
Gomez-Montes developed a score including TV/MV ratio ≤ 0.83, PV/AV ratio ≤ 0.75, tricuspid inflow duration/cardiac cycle length ≤ 36.5% and RV/LV length ratio ≤ 0.64. Three fulfilled criteria predict non-BV outcome with sensitivity of 100%, specificity of 92%, and both 100% if four criteria are fulfilled [21].
The only patient with prenatal pulmonary valvuloplasty in our cohort had TV/MV ratio of 0.53 and had BV outcome. After our cutoff of 0.62, non-BV outcome would have been predicted.
As already described by others in literature [6, 10, 12] we found significant more patients with VCAC within the non-BV outcome group. However, two patients with VCAC had BV outcome and one 1.5 circulation. Gardiner et al. also had one patient with VCAC and BV outcome and another with potential for BV outcome. According to Maeno et al., only 50% of VCAC are resulting in RV-depended circulation [9]. Successful right ventricular decompression depends on coronary arterial anatomy. Stenosis of a single coronary artery may not preclude successful right ventricular decompression; whereas, involvement of both arteries seems to be a contraindication. [22]
Prenatal detection of VCAC is challenging. Maeno et al. reported 7/13 (53.8%) correct prenatal diagnoses of VCAC. [9] In our cohort, prenatal diagnosis was failed in five cases of 34 (14.7%) and in three cases, VCAC were presumed prenatally, but not confirmed postnatally. TI-Vmax > 2.5 was associated with BV outcome and absence with UV outcome as described before [5, 7, 8, 20]. In our cohort, 75% with BV outcome had TI-Vmax > 2.5 m/s.
There exist few data concerning growth of RV structures during gestation.
While some showed significantly higher in-utero growth rate of TV in patients with BV outcome [6], we found also a tendency, but without significance.
In a recently published cohort of Tulzer et al. with 23 foetuses with intrauterine, pulmonary valvuloplasty at a median of 28 + 4 weeks, corresponding to our interval 2, the Roman score was retrospectively applied in 20/23 (87.0%) patients with prenatal valvuloplasty [23]. In ten of 20 (50%), UV outcome was predicted by applying this score. After pulmonary valvuloplasty, BV outcome was achieved in five of those 10 (50%) foetuses, in two circulation was yet undetermined, in two 1.5 circulation was reached and one with failed prenatal intervention had UV circulation [23].
However, in ten of 23 (43.5%) foetuses with prenatal valvuloplasty, the Roman score predicted BV outcome.
TV/MV ratio before intervention in the Tulzer cohort ranged from 0.62 to 0.97 (median 0.69). According to our TV/MV ratio, BV outcome would have also been predicted in these ten fetuses.
Knowing the fact that it is difficult to compare different cohorts, we applied the inclusion criteria of Tulzer for foetal intervention to our cases with predicted non-BV outcome after our score. Only four of 9 in interval 2 with predicted non-BV outcome would have been candidates for intervention. However, two of them reached BV outcome postnatally, one after intrauterine intervention and one without intervention. In interval 3, five of 14 would have been candidates for intervention. Two of them reached BV outcome and one 1.5 circulation without intervention, questioning the present selection criteria for foetal intervention. This issue was subject of controversy in the paper of the International cardiac intervention registry [24]. Suboptimal selection criteria for foetal aortic valvuloplasty have also been addressed by Gardiner et al. [25] It is difficult to apply published scores to different populations, because outcome also depends on postnatal decision-making and surgical performance which differs between centres. According to Tworetzky et al., foetal pulmonary valvuloplasty in PAIVS seems to improve right heart growth and postnatal outcomes for fetuses with moderate right heart hypoplasia, but there is an important learning curve [11]. It is an invasive procedure with risk of prematurity. Criteria for foetuses who might have a benefit must further be evaluated.
A limitation of our analysis is limitation of available measurements, small measurements with considerable error, combination of prospective and retrospective measurements and short-term postnatal outcome. Due to the retrospective data analysis, the lost-for-follow-up rate of patients is quite high and some echocardiographic variables included are without real blinding to outcomes (TI, presence of VCAC); so, there is potential for bias.
Conclusion
To our knowledge, we evaluated one of the largest prenatal cohorts with PAIVS/CPS. TV/MV ratio is a simple single parameter with high predictive value. Prenatal diagnosis of VCAC is feasible in centres with expertise and is associated with non-BV outcome as already described before. Including the most predictive parameters, TV/MV ratio, TI-Vmax < 2.5 m/s and presence of VCAC into a scoring system, non-BV outcome was correctly predicted in all our cases if more than one criteria were fulfilled. Criteria for foetal intervention should be evaluated further in a prospective manner.
References
Daubeney PEF, Sharland GK, Cook AC, Keeton BR, Anderson RH, Webber SA (1998) Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. Circulation 98(6):562–566
Daubeney PEF, Wang D, Delany DJ, Keeton BR, Anderson RH, Slavik Z et al (2005) Pulmonary atresia with intact ventricular septum: Predictors of early and medium-term outcome in a population-based study. J Thorac Cardiovasc Surg. 130(4):1–9
Dyamenahalli U, McCrindle BW, McDonald C, Trivedi KR, Smallhorn JF, Benson LN et al (2004) Pulmonary atresia with intact ventricular septum: management of, and outcomes for, a cohort of 210 consecutive patients. Cardiol Young. 14(3):299–308
Schwedler G, Lindinger A, Lange PE, Sax U, Olchvary J, Peters B et al (2011) Frequency and spectrum of congenital heart defects among live births in Germany : a study of the competence network for congenital heart defects. Clin Res Cardiol 100(12):1111–1117
Gardiner HM, Belmar C, Tulzer G, Barlow A, Pasquini L, Carvalho JS et al (2008) Morphologic and functional predictors of eventual circulation in the fetus with pulmonary atresia or critical pulmonary stenosis with intact septum. J Am Coll Cardiol. 51(13):1299–1308
Salvin J, McElhinney D, Colan S, Gauvreau K, del Nido P, Jenkins K et al (2006) Fetal tricuspid valve size and growth as predictors of outcome in pulmonary atresia with intact ventricular septum. Pediatrics 118(2):e415–e420
Peterson R, Levi D, Williams R, Lai W, Sklansky M, Drant S (2006) Echocardiographic predictors of outcome in fetuses with pulmonary atresia with intact ventricular septum. J Am Soc Echocardiogr. 19(11):1393–1400
Iacobelli R, Pasquini L, Toscano A, Raimondi F, Michielon G, Sanders SP (2008) Role of tricuspid regurgitation in fetal echocardiographic diagnosis of pulmonary atresia with intact ventricular septum. Ultrasound Obstet Gynecol 32(1):31–35
Maeno YV, Boutin C, Hornberger LK, Mccrindle BW, Gladman G (1999) Prenatal diagnosis of right ventricular outflow tract obstruction with intact ventricular septum, and detection of ventriculocoronary connections. Heart 81(6):661–668
Roman KS, Fouron J-C, Nii M, Smallhorn JF, Chaturvedi R, Jaeggi ET (2007) Determinants of outcome in fetal pulmonary valve stenosis or atresia with intact ventricular septum. Am J Cardiol. 99(5):699–703
Tworetzky W, Doff B, Marx GR, Benson CB, Brusseau R, Morash D et al (2009) In Utero Valvuloplasty for pulmonary atresia with hypoplastic right ventricle: techniques and outcomes. Pediatrics 124(3):e510–e518
Gottschalk I, Strizek B, Menzel T, Herberg U, Breuer J, Brockmeier K et al (2020) Severe pulmonary stenosis or atresia with intact ventricular septum in the fetus the natural history. Fetal Diagn Ther. 47(5):420–428
Paladini D, Rustico M, Todros T, Palmieri S, Gaglioti P, Benettoni A et al (1996) Conotruncal anomalies in prenatal life. Ultrasound Obstet Gynecol. 8(4):241–246
Shinebourne E, Rigby M, Carvalho J (2008) Pulmonary atresia with intact ventricular septum: from fetus to adult: congenital heart disease. Hear 94(10):1350–1357
Carvalho JS, Allan LD, Chaoui R, Copel JA, DeVore G, Hecher K et al (2013) ISUOG Practice guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol 41(3):348–359
Schneider C, McCrindle BW, Carvalho JS, Hornberger LK, McCarthy KP, Daubeney PEF (2005) Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol 26(6):599–605
Quartermain MD, Glatz AC, Goldberg DJ, Cohen MS, Elias MD, Tian Z et al (2013) Pulmonary outflow tract obstruction in fetuses with complex congenital heart disease: predicting the need for neonatal intervention. Ultrasound Obstet Gynecol 41(1):47–53
Schneider AW, Blom NA, Bruggemans EF, Hazekamp MG (2014) More than 25 years of experience in managing pulmonary atresia with intact ventricular septum. Ann Thorac Surg. 98(5):1680–1686
Peng QH, Zhou QC, Zeng S et al (2009) Evaluation of regional left ventricular longitudinal function in 151 normal fetuses using velocity vector imaging. Prenat Diagn 29(12):1149–1155
Lowenthal A, Kipps AK, Brook M (2014) Prenatal tricuspid valve size as a predictor of postnatal outcome in patients with severe pulmonary stenosis or pulmonary atresia with. Fetal Diagn Ther 35(2):101–107
Gómez-Montes E, Herraiz I, Mendoza A, Albert L, GalindoHernández-García JMA (2011) Pulmonary atresia / critical stenosis with intact ventricular septum: prediction of outcome in the second trimester. Prenat Diagn. 31(4):372–379
Giglia TM, Mandell VS, Connor ARJEM Jr, Lock JE (1990) Diagnosis and management of right ventricle-dependent coronary circulation in pulmonary atresia with intact ventricular septum. Circulation 86(5):1516–1528
Tulzer A, Arzt W, Gitter R, Prandstetter C, Grohmann E, Mair R et al (2018) Immediate effects and outcome of in-utero pulmonary valvuloplasty in fetuses with pulmonary atresia with intact ventricular septum or critical pulmonary stenosis. Ultrasound Obstet Gynecol. 52(2):230–237
Moon-Grady A, Morris S, Belfort M, Chmait R, Dangel J, Devlieger R et al (2015) International fetal cardiac intervention registry: a worldwide collaborative description and preliminary outcomes. J Am Coll Cardiol 66(4):388–399
Gardiner HM, Kovacevic A, Tulzer G, Sarkola T, Herberg U (2016) Natural history of 107 cases of fetal aortic stenosis from a European multicenter retrospective study. Ultrasound Obstet Gynecol 48(3):373–381
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Protocol/project development: WA, A-FR, KM, JC. Data collection: WA, MN, KA, DJ, RJ, RS, EC, BI, VC, SJ, GO, KM, JT. Data analysis: WJ, WA, A-FR. Manuscript writing/editing: WA, A-FR.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolter, A., Markert, N., Wolter, J.S. et al. Natural history of pulmonary atresia with intact ventricular septum (PAIVS) and critical pulmonary stenosis (CPS) and prediction of outcome. Arch Gynecol Obstet 304, 81–90 (2021). https://doi.org/10.1007/s00404-020-05929-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05929-0