Abstract
Purpose
To evaluate the performance of first trimester maternal serum glycosylated (Sambucus nigra lectin-reactive) fibronectin in prediction of gestational diabetes mellitus (GDM).
Methods
In this case–control study, first trimester maternal serum glycosylated fibronectin and fibronectin were measured in 19 women who consequently developed GDM and in 59 control women with normal pregnancy outcomes. Adiponectin was used as a reference protein to evaluate relation of glycoprotein to SNA-lectin-reactive assay format. Samples were taken during gestational weeks 9+6–11+6. Data concerning GDM was obtained from the National Institute for Health and Welfare, which records the pregnancy outcomes of all women in Finland.
Results
There was no difference in maternal serum glycosylated fibronectin concentrations between women with consequent GDM [447.5 μg/mL, interquartile range (IQR) 254.4–540.9 μg/mL] and control women (437.6 μg/mL, IQR 357.1–569.1 μg/mL). Maternal serum fibronectin levels were significantly lower in GDM group (224.2 μg/mL, IQR 156.8–270.6 μg/mL), compared to the control group (264.8 μg/mL, IQR 224.6–330.6 μg/mL, p < 0.01). There was no difference in assay formats for adiponectin.
Conclusion
There was no association between first trimester maternal serum glycosylated (SNA-reactive) fibronectin and GDM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a growing risk for both maternal and fetal health as its prevalence is increasing worldwide. In Finland, the prevalence of GDM was 15.6% in 2017 and 14.1% of women were obese [body mass index (BMI) ≥ 30 kg/m2] before pregnancy [1]. Increasing prevalence is worrisome as the adverse effects of GDM are not confined to pregnancy since it can also affect the later health of both mothers and children [2, 3]. Healthy lifestyle changes in diet and exercise can reduce the risk of GDM and the effect is stronger the earlier the intervention is made [4]. Currently, however, in most cases GDM is diagnosed with an abnormal oral glucose tolerance test (OGTT) only at gestational weeks 24–28. There is a need for an effective first trimester screening method for GDM to enable robust intervention.
Previous studies have shown that maternal serum biomarkers are altered already in the first trimester of pregnancy in women who subsequently develop GDM. Studied markers include metabolites such as glycine and arginine, fatty acids, sex hormone binding globulin (SHBG), inflammatory markers, adipocyte-derived markers, placenta-derived markers, placental exomes and glycosylated (Sambucus nigra lectin-reactive) fibronectin [5,6,7,8,9,10,11,12].
Some of the reports have been promising and marker combinations have been suggested to have up to 72% detection rate (DR) for a 20% false positive rate (FPR) [10]. However, published reports have been partly controversial and concerning maternal serum glycosylated fibronectin, that was reported to have excellent performance, there are only few published reports suggesting its usefulness as first trimester predictor of subsequent GDM [6, 13]. Fibronectin is a large dimeric glycoprotein that is expressed in various cell and tissue types and participates in multiple functions such as cell adhesion, growth, migration and differentiation. Cellular form of fibronectin is synthetized by endothelial cells, fibroblasts and smooth muscle cells. Fibronectin is one of the most reliable proteins that can be estimated as a plasma indicator protein for endothelial function and related pathological disorders [14,15,16]. Significant elevation of circulating fibronectin have been reported in various metabolic syndromes associated with endothelial function, such as diabetes [17,18,19]. The change in the levels of serum or plasma cellular fibronectin may reflect the extent of matrix changes and vessel wall damage in patients with diabetes. Although various forms of fibronectin have been implicated to have a role in metabolic disorders like diabetes the specific glycosylated version in question has only been investigated in just few studies [16]. Beside GDM, glycosylated plasma fibronectin has been indicated as an early predictor for pre-eclampsia, another major pregnancy disorder [20, 21].
Glycosylation of protein is an enzymatic process (e.g., various transferases), whereas related glycation process is a non-enzymatic process, where excess sugars are removed from body using so called amadori intermediate products, glycated hemoglobin (HbA1c) is one example of such protein.
Adiponectin is another glycoprotein that has been associated with GDM [22]. Adiponectin is a protein hormone that has a role in regulating glucose levels and fatty acid metabolism. In this study adiponectin was used as a comparator protein to evaluate SNA-lectin-based assay performance to direct sandwich immunoassay.
The aim of this study was to reevaluate the screening performance of first trimester maternal serum glycosylated fibronectin for GDM. For this purpose, the difference between first trimester glycosylated fibronectin values were compared between women with and without GDM.
Materials and methods
The data for this retrospective case–control study was retrieved from first trimester combined screening program in Northern Finland during the study period of 1.1.2007–31.12.2011. Participation in combined screening program is voluntary. Maternal serum glycosylated fibronectin levels were measured from the frozen combined screening samples of a subset of women including 19 women who subsequently developed GDM and 59 control women who were not diagnosed with GDM. Small sample size was assumed to be sufficient based on high marker performance in original publication [16]. Power analysis assuming 80% sensitivity showed that with 20 cases the exact 95% confidence interval is 56.3–94.3%. As we did not aim to refute but to confirm previously reported performance level the small sample size was considered to be sufficient for this.
Women with multiple gestation or other pregnancy complications than GDM, were excluded from the study. There were no ethnic differences between the groups. Data concerning GDM was obtained from the National Institute for Health and Welfare, which records the outcome of all live births and stillbirths with a gestational age of 22+° or more or a birth weight of 500 g or more in the country.
A 2-h 75 g OGTT during gestational weeks 24–28 or at 12–16 gestational weeks was used to diagnose GDM. Diagnostic cut-off values were ≥ 5.3 mmol/L (fasting blood glucose), ≥ 10.0 mmol/L (1 h) and ≥ 8.6 mmol/L (2 h). If one or more of the values were abnormal the woman was diagnosed as having GDM. Screening for GDM is universal in Finland but not all women undergo it since the OGTT is not recommended if the primipara is under 25 years with no family history of type 2 diabetes mellitus (T2DM) and with pre-pregnancy BMI of 18.5–25 or multipara is under 40 years with no previous GDM or macrosomia and with pre-pregnancy BMI under 25 kg/m2. OGTT during gestational weeks of 12–16 is recommended for women in increased risk of GDM (BMI ≥ 35, a previous GDM, glucosuria in the first trimester, polycystic ovary syndrome or a first-degree relative with T2DM). If early OGTT is negative, it is repeated at gestational weeks 24–28. Women with no testing for GDM were not included in this study.
We developed DELFIA research assays for fibronectin and glycosylated fibronectin. Normal fibronectin assay was done by coating 96-well plates with mouse monoclonal IgG antibody against human fibronectin (MAB1918, R&D Systems, Abingdon, UK). Sheep polyclonal Eu-N1-labeled Anti-hFibronectin antibody was used as a tracer (AF1918, R&D Systems, Abingdon, UK). To reduce unspecific binding in the assay for glycosylated (SNA-reactive) fibronectin the same primary coating antibody was pre-treated with Remove iT Endo S (New England BioLabs, Ipswich, MA, USA) reagent. Endo S is an endoglycosidase with a high specificity for removing N-linked glycans from the heavy chain of native IgG. Detection was done using biotinylated Sambucus Nigra Elderberry Bark Lectin (SNA) (Vector Laboratories, Burlingame, CA, USA) and Eu-SA (PerkinElmer, Waltham, MA, USA). Calibrators for both assays were made from human plasma derived fibronectin (1918-FN, R&D Systems, Abingdon, UK). Samples were diluted 1:2000 and 1:1000 for fibronectin and glycosylated (SNA-reactive) fibronectin assays, respectively. Run control coefficient of variation (CV) was 2.4% for both assays.
As a comparator for lectin-based assays we also developed DELFIA research assays for adiponectin and glycosylated adiponectin. Standard adiponectin assay was done by coating 96-well plates with mouse monoclonal IgG antibody against human adiponectin (MAB10651, R&D Systems, Abingdon, UK). Mouse monoclonal Eu-N1-labeled anti-hAdiponectin antibody was used as a tracer (MAB1065, R&D Systems, Abingdon, UK). To reduce unspecific binding in the assay for glycosylated (SNA-reactive) adiponectin the primary coating antibody was again pre-treated with Remove iT Endo S (New England BioLabs, Ipswich, MA, USA) reagent. Detection was done using biotinylated Sambucus Nigra Elderberry Bark Lectin (SNA) (Vector Laboratories, Burlingame, CA, USA) and Eu-SA (PerkinElmer, Waltham, MA, USA). Calibrators for both assays were made from recombinant human adiponectin (1065-AF, R&D Systems, Abingdon, UK). Samples were diluted 1:100 for adiponectin and glycosylated (SNA-reactive) adiponectin assays. Run control CV% was 4.0% for both assays.
Spotfire (TIBCO) was used to statistically analyze the results. Linear regression analysis was used to evaluate assay correlations. For continuous variables analysis of variance (ANOVA) and Chi square for categorical variables was used. Statistical significance was set for p value of < 0.05. Univariate logistic regression was done to create a ROC plot that was used to assess screening performance.
Results
The mean age of women in the study group was 30.8 years (range 28.5–33.1) and 30.1 years (26.4–34.3) in the control group at sampling. There were no significant differences in gestational age at sampling, maternal weight or smoking habits. Maternal serum fibronectin levels were significantly lower (p = 0.007) in GDM group, 224.2 μg/mL (interquartile range (IQR) 156.8–270.6 μg/mL), compared to the control group 264.8 μg/mL (224.6–330.6 μg/mL, p < 0.01). There was, however, no statistical difference between the two groups in glycosylated fibronectin levels: 447.5 μg/mL (IQR 254.4–540.9 μg/mL) in the GDM group and 437.6 μg/mL (IQR 357.1–569.1 μg/mL) in the control group. Table 1 enlists maternal characteristics, OGTT results and median fibronectin and glycosylated fibronectin levels with IQRs in GDM and control group.
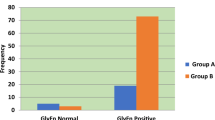
OGTT results in GDM and control groups are presented in Fig. 1. The difference at each time point (0, 1 and 2 h) was statistically significant (p < 0.01 or p < 0.001) between the two groups. Figure 2 shows that there was a high correlation (R2 ~ 0.8) between glycosylated fibronectin (SNA-based assay) and normal fibronectin (antibody-based assay). Indicating that glycosylation detected by SNA-lectin is not totally independent from normal glycosylation pattern when measuring protein through immunogenic epitopes. Correlation between adiponectin and SNA-reactive adiponectin was low (R2 < 0.1) in both, GDM and control groups.
The difference in glycosylated fibronectin concentrations between the GDM and control women did not differ statistically significantly in different BMI subgroups. In the normal BMI group of 20–25 kg/m2, the glycosylated fibronectin levels were 433.9 μg/mL (IQR 365.4–546.6, n = 22) in the control group and 407.6 μg/mL (IQR 344.7–473.6, n = 4) in the GDM group. In the obese BMI group of 30–35 kg/m2, the concentrations were 371.2 μg/mL (IQR 304.3–548.2, n = 8) in the control group and 649.9 μg/mL (IQR 421.2–770.1, n = 6) in the GDM group (Fig. 3a). Smoking did not alter the glycosylated fibronectin levels statistically significantly between the control and GDM groups: the levels for smokers in these groups were 379.3 μg/mL (IQR 263.8–391.0) and 366.4 μg/mL (IQR 293.0–407.6), respectively, and for the non-smokers in the same control and GDM groups, 467.5 μg/mL (IQR 390.2–594.0) and 483.6 μg/mL (IQR 266.9–563.0), respectively (Fig. 3b).
a Glycosylated fibronectin concentrations in BMI subgroups of 20–25 and 30–35 kg/m2 in the study and the control group. b Effect of smoking on concentrations of glycosylated fibronectin in the control and GDM groups. c Multiple of median (MoM) values for fibronectin and glycosylated fibronectin between control and GDM groups. d Multiple of median (MoM) values for adiponectin and glycosylated adiponectin between control and GDM groups
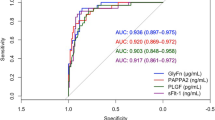
Analyte concentrations were normalized to multiple of median (MoM) values so the possible effect from underlying co-factors, such as gestational age, maternal age, weight, smoking status, could be eliminated. Use of MoM values are widely used in prenatal risk screening, e.g., for aneuploidies and pre-eclampsia. MoM values of fibronectin (p = 0.02) and glycosylated fibronectin (p = 0.61) as well as adiponectin and glycosylated adiponectin between controls and GDM women are presented in Fig. 3c, d. Logistic regression model was used to evaluate the screening performance of both glycosylated fibronectin and fibronectin for GDM (Fig. 4). With the same sensitivity of 42.1%, fibronectin had better specificity than glycosylated fibronectin, 91.5 and 81.5%, respectively. In the BMI group of 30–35 kg/m2, glycosylated fibronectin had a better screening performance: for a sensitivity of 67% the specificity was 67%.
Median adiponectin concentration levels were 141 μg/mL for control samples and 123 μg/mL for GDM samples and were approximately 40% lower than concentrations measured using SNA-lectin assay version, 201 and 173 μg/mL, respectively. Multiple of median (MoM) values of adiponectin and glycosylated adiponectin between controls and GDM women are presented in Fig. 7. Logistic regression model was not evaluated for the screening performance as neither of the adiponectin assays did not show any significance on predicting GDM in this sample set.
Discussion
In this study, there was no statistical difference in glycosylated fibronectin concentrations in the first trimester maternal serum samples between the GDM group and control group. Fibronectin concentrations were significantly lower in the GDM group compared to controls. BMI and smoking affected glycosylated fibronectin levels. Smokers tended to have lower glycosylated fibronectin concentrations compared to non-smokers and the difference was seen within both GDM and control group. Increase in BMI enhanced glycosylated fibronectin concentration in GDM group but not in the control group. These differences between BMI and smoking subgroups, however, did not reach statistical significance within the present design and statistical assumptions.
Novel screening markers for GDM are needed as currently GDM is mostly diagnosed only in the second trimester of the pregnancy. In Europe, there is a lack of consensus regarding GDM screening and practices and policies vary even within countries [23, 24]. The early detection and treatment for GDM has benefits for both mother and the offspring [25,26,27]. There is no clear threshold in maternal glucose levels after which risk of pregnancy complications start to enhance but the increase is continuous [28]. Inadequacies in current screening have led to the search of maternal serum markers by which an earlier and more efficient detection of GDM could be reached.
The purpose of this study was to evaluate the screening performance of first trimester maternal serum glycosylated fibronectin as a marker for GDM. Rasanen et al. (2013) found in their study a significant difference in glycosylated fibronectin concentrations between the GDM group (132 ± 36 mg/L, n = 90) and the control group (80 ± 4.0 mg/L, n = 92, p < 0.001) [6]. In this study, there was no statistically significant difference between the groups, although glycosylated fibronectin concentrations were slightly higher in the GDM group compared to controls. There was a statistically non-significant increase in the GDM group compared to control group in women with BMI of 30–35 kg/m2 suggesting that glycosylated fibronectin might be a better marker in obese women. However, the number of obese women was too small; six women with GDM and eight control women.
Rasanen et al. (2013) used a fibronectin monoclonal antibody (MAB1918) as primary antibody in enzyme-linked immunoassay using a Konelab 60i Clinical Chemistry Analyzer. They also used biotin-conjugated Sambucus nigra lectin (Vector Labs) in the process. In this study, the same primary coating antibody was pre-treated with Remove iT Endo S to reduce unspecific binding to glycosylated fibronectin. Different laboratory techniques might explain differences in the results. All forms of fibronectin are glycosylated but here the term glycosylated fibronectin refers to specific sialyated glucose that SNA lectin recognizes. Concentration levels from normal pregnancies were not directly comparable to concentration in Rasanen et al. (2013) which is due to lack of international standards preparation for calibration, although primary analyte detecting reagents were same.
Nagalla et al. (2015) suggested using fibronectin-SNA as a single marker test for GDM in the first trimester of pregnancy. In contrast to results of this study, glycosylated fibronectin (fibronectin-SNA) levels were significantly higher in GDM group (n = 15) compared to controls (n = 14). There was no difference in maternal pre-pregnancy BMI between the GDM and control groups [13].
Limited number of studies have assessed the role of glycosylated fibronectin in screening for GDM. The published studies are limited in the number of cases as is the current study. There is an ongoing prospective study using maternal serum glycosylated fibronectin and/or OGTT 75 g at 12–15 gestational weeks as a predictor of subsequent GDM [30]. Meanwhile, this study provides information on the screening performance of glycosylated fibronectin in women with GDM. This study also evaluated glycosylated fibronectin levels in different BMI subgroups.
Conclusions
According to our results, neither first trimester maternal serum glycosylated fibronectin nor serum fibronectin are effective in screening for GDM. Further larger studies in prospective settings are needed to evaluate first trimester glycosylated fibronectin as a screening method for GDM alone and in combination with other markers.
Abbreviations
- BMI:
-
Body mass index
- CV:
-
Coefficient of variation
- DR:
-
Detection rate
- GDM:
-
Gestational diabetes mellitus
- FPR:
-
False positive rate
- IQR:
-
Interquartile range
- MoM:
-
Multiples of the median
- OGTT:
-
Oral glucose tolerance test
- SHBG:
-
Sex hormone binding globulin
- T2DM:
-
Type 2 diabetes mellitus
References
National Institute for Health and Welfare. Perinatal statistics: parturients, deliveries and newborns (2012) Statistical Report 24/2013. National Institute for Health and Welfare, Helsinki
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373:1773–1779. https://doi.org/10.1016/S0140-6736(09)60731-5
Damm P (2009) Future risk of diabetes in mother and child after gestational diabetes mellitus. Int J Gynaecol Obstet 104:S25–S26. https://doi.org/10.1016/j.ijgo.2008.11.025
Rono K, Stach-Lempinen B, Klemetti MM, Kaaja RJ, Poyhonen-Alho M, Eriksson JG, Koivusalo SB, Andersson S, Huvinen E, RADIEL group (2014) Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth 14(14):70. https://doi.org/10.1186/1471-2393-14-70
Kotronen A, Velagapudi VR, Yetukuri L, Westerbacka J, Bergholm R, Ekroos K, Makkonen MR, Taskinen MO, Yki-Jarvinen H (2009) Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia 52:684–690. https://doi.org/10.1007/s00125-009-1282-2
Rasanen JP, Snyder CK, Rao PV, Mihalache R, Heinonen S, Gravett MG, Roberts C, Nagalla S (2013) Glycosylated fibronectin as a first-trimester biomarker for prediction of gestational diabetes. Obstet Gynecol 122:586–594. https://doi.org/10.1097/AOG.0b013e3182a0c88b
Syngelaki A, Kotecha R, Pastides A, Wright A, Nicolaides KH (2015) First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism 64:1485–1489. https://doi.org/10.1016/j.metabol.2015.07.015
Iliodromiti S, Sassarini J, Kelsey TW, Lindsay RS, Sattar N, Nelson SM (2016) Accuracy of circulating adiponectin for predicting gestational diabetes: a systematic review and meta-analysis. Diabetologia 59:692–699. https://doi.org/10.1007/s00125-015-3855-6
Migda M, Migda MS, Migda B, Krzyżanowska P, Wender-Ożegowska E (2016) Components of metabolic syndrome in the first trimester of pregnancy as predictors of adverse perinatal outcome. Ginekol Pol 87:644–650. https://doi.org/10.5603/GP.2016.0060
Nevalainen J, Sairanen M, Appelblom H, Gissler M, Timonen S, Ryynänen M (2016) First-trimester maternal serum amino acids and acylcarnitines are significant predictors of gestational diabetes. Rev Diabet Stud 13:236–245. https://doi.org/10.1900/RDS.2016.13.236
Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Longo S, Duncombe G, Mitchell MD, Rice GE, Illanes SE (2016) Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes 65:598–609. https://doi.org/10.2337/db15-0966
Syngelaki A, Visser GH, Krithinakis K, Wright A, Nicolaides KH (2016) First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism 65:131–137. https://doi.org/10.1016/j.metabol.2015.10.029
Nagalla SR, Snyder CK, Michaels JE, Laughlin MJ, Roberts CT, Balaji M, Balaji M, Seshiah V, Rao PV (2015) Maternal serum biomarkers for risk assessment in gestational diabetes. A potential universal screening test to predict GDM status. Indian J Endocrinol Metab 19:155–159. https://doi.org/10.4103/2230-8210.140226
Peters JH, Ginsberg MH, Bohl BP, Sklar LA, Cochrane CG (1986) Intravascular release of intact cellular fibronectin during oxidant-induced injury of the in vitro perfused rabbit lung. J Clin Invest 78:1596–1603
Ruoslahti E (1988) Fibronectin and its receptors. Annu Rev Biochem 57:375–413
Magnusson MK, Mosher DF (1998) Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol 18:1363–1370
Peters JH, Ginsberg MH, Case CM, Cochrane CG (1988) Release of soluble fibronectin containing an extra type III domain (ED1) during acute pulmonary injury mediated by oxidants or leukocytes in vivo. Am Rev Respir Dis 138:167–174
Peters JH, Maunder RJ, Woolf AD, Cochrane CG, Ginsberg MH (1989) Elevated plasma levels of ED1+ (“cellular”) fibronectin in patients with vascular injury. J Lab Clin Med 113:586–597
Lockwood CJ, Peters JH (1990) Increased plasma levels of ED1+ cellular fibronectin precede the clinical signs of preeclampsia. Am J Obstet Gynecol 162:358–362
Lazarchick J, Stubbs TM, Romein L, Van Dorsten JP, Loadholt CB (1986) Predictive value of fibronectin levels in normotensive gravid women destined to become preeclamptic. Am J Obstet Gynecol 154:1050–1052. https://doi.org/10.1016/0002-9378(86)90748-9
Rasanen J, Quinn MJ, Laurie A, Bean E, Roberts CT Jr, Nagalla SR, Gravett MG (2015) Maternal serum glycosylated fibronectin as a point-of-care biomarker for assessment of preeclampsia. Am J Obstet Gynecol 212:82.e1–9. https://doi.org/10.1016/j.ajog.2014.07.052
Sweeting AN, Wong J, Appelblom H, Ross GP, Kouru H, Williams PF, Sairanen M, Hyett JA (2019) A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn Ther 45:76–84. https://doi.org/10.1159/000486853
Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, Dunne VF, Teramo K (2012) Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening: a review. Diabet Med 29:844–854. https://doi.org/10.1111/j.1464-5491.2011.03541.x
Lindqvist M, Persson M, Lindkvist M, Mogren I (2014) No consensus on gestational diabetes mellitus screening regimes in Sweden: pregnancy outcomes in relation to different screening regimes 2011 to 2012, a cross-sectional study. BMC Pregnancy Childbirth 31(14):185. https://doi.org/10.1186/1471-2393-14-185
Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 352:2477–2486. https://doi.org/10.1056/NEJMoa042973
Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Sciscione A, Catalano P (2009) A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 361:1339–1348. https://doi.org/10.1056/NEJMoa0902430
Sacks DA (2009) Gestational diabetes–whom do we treat? N Engl J Med 1(361):1396–1398. https://doi.org/10.1056/NEJMe0907617
The HAPO Study Cooperative Research Group (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991–2002. https://doi.org/10.1056/NEJMoa0707943
Huhn EA, Fischer T, Göbl CS, Todesco Bernasconi M, Kreft M, Kunze M, Schoetzau A, Dolzlmuller E, Eppel W, Husslein P, Ochsenbein-Koelble N, Zimmermann R, Baz E, Prompeler H, Bruder E, Hahn S, Hoesli I (2016) Screening of gestational diabetes mellitus in early pregnancy by oral glucose tolerance test and glycosylated fibronectin: study protocol for an international, prospective, multicentre cohort trial. BMJ Open. https://doi.org/10.1136/bmjopen-2016-012115
Acknowledgements
Open access funding provided by University of Oulu including Oulu University Hospital.
Funding
PerkinElmer (Turku, Finland) sponsored the reagents for fibronectin, glycosylated fibronectin and adiponectin assays.
Author information
Authors and Affiliations
Contributions
JN: protocol/project development, data collection or management, manuscript writing/editing. JA: data collection or management, manuscript writing. HA, TK, HK, MS: data collection or management, data analysis, manuscript writing/editing. MG: data collection or management. MR: protocol/project development, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
Heidi Appelblom, MSc, is a research scientist, Teemu Korpimaki, PhD, is a senior chemist, Heikki Kouru, PhD, is a senior statistician and Mikko Sairanen, PhD, is a scientific liaison working for PerkinElmer, Turku, Finland. PerkinElmer is a supplier of biochemical products for early pregnancy trisomy screening performed in Oulu University Hospital. The other authors have no potential conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
Ethical approval
The Institutional Ethics Committee of Oulu University Hospital and the National Institute for Health and Welfare approved this study.
Informed consent
Informed consent was waived by the IRB as this was a retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alanen, J., Appelblom, H., Korpimaki, T. et al. Glycosylated fibronectin as a first trimester marker for gestational diabetes. Arch Gynecol Obstet 302, 853–860 (2020). https://doi.org/10.1007/s00404-020-05670-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05670-8