Abstract
Background
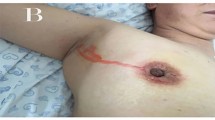
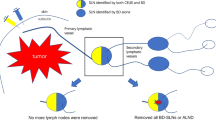
Recent studies show that contrast-enhanced ultrasonography (CEUS) using SonoVue has the potential to improve the performance of sentinel lymph node biopsy (SLNB) in patients with early breast cancer. However, the evidence of SLNB using CEUS in patients converting from cN1 to cN0 after neoadjuvant chemotherapy (NAC) is lacking. The aim of this prospective study is to evaluate the feasibility of CEUS using SonoVue for the identification of sentinel lymph node (SLN) and the value of the combination of CEUS and blue dye (BD) for SLNB in patients converting from cN1 to cN0 following NAC.
Methods
Patients with cytology-proven node positive breast cancer at the initial diagnosis (stage T1-T3N1M0) from January 2018 to January 2019, underwent NAC. Patients converting from cN1 to cN0 following NAC were enrolled and randomized into two groups for SLNB: the combination method group using CEUS and BD together, and the single BD method group. Then all patients underwent complete axillary lymph node dissection (ALND) and primary breast surgery. Compared with the final pathological results, the identification rate, sensitivity, specificity, accuracy, false negative rate, negative predictive value, positive predictive value were recorded and compared between two methods.
Results
A total of 400 patients with stage T1-T3N1M0 disease underwent NAC between January 2018 to January 2019, among which 134 (33.5%) patients had clinically negative node confirmed by imaging after NAC and randomized into two groups. Each group included 67 cases. In the combination method group, contrast-enhanced lymphatic vessels in 66 cases of 67 were clearly visualized by US soon after the periareolar injection of SonoVue and the SLNs were accurately localized. The identification rate of the combination method was 98.5%%, which was significantly higher than 83.6% (56/67) using the single BD method. The mean numbers of SLNs identified by the combination method was higher than that by the single BD method. Compared with pathological diagnosis, sensitivity, specificity, accuracy, the positive predictive value, the negative predictive value, and the FNR of the combingation method were 84.4%, 100%, 89.4%, 100%, 75%, and 15.6%, respectively. In contrast, sensitivity, specificity, accuracy, the positive predictive value, the negative predictive value, and the FNR using single blue dye were 73.9%, 100%, 89.3%, 100%, 84.6%, and 26.1%, respectively. The FNR using the combination method was significantly lower than that using single BD.
Conclusion
Identification of SLNs in patients converting from cN1 to cN0 following NAC by CEUS is a technically feasible. The combination of CEUS and BD is more effective than BD alone for SLNB in patients converting from cN1 to cN0 following NAC.


Similar content being viewed by others
References
Lyman GH, Somerfield MR, Giuliano AE (2017) Sentinel lymph node biops-y for patients with early-stage breast cancer: 2016 American Society of ClinicalOncology clinical practice guideline update summary. J Oncol Pract 13:196–198
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM et al (2010) Sentinel-lymphnode resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer:overallsurvival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11:927–933
Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, Skelly JM, Harlow SP, Weaver DL, Mamounas EP et al (2010) Morbidity results from the NSABP B-32 trial comparing sentinel lymphn-ode dissection versus axillary dissection. J Surg Oncol 102:111–118
Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I et al (2006) Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 98:599–609
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M (2011) Axillary dissection vs no xillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M et al (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 14:297–305
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL, Wolmark N et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15:2483–2493
Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, Bear HD, Caldwell CB, Walker AP, Mikkelson WM et al (2005) Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 23:2694–2702
van Deurzen CH, Vriens BE, Tjan-Heijnen VC, van der Wall E, Albregts M, van Hilligersberg R, Monninkhof EM, van Diest PJ (2009) Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer 45:3124–3130
Mocellin S, Goldin E, Marchet A, Nitti D (2016) Sentinel node biopsy performance after neoadjuvant chemotherapy in locally advanced breast cancer: a systematic review and meta-analysis. Int J Cancer 138:472–480
Lee S, Kim EY, Kang SH, Kim SW, Kim SK, Kang KW, Kwon Y, Shin KH, Kang HS, Ro J et al (2007) Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients. Breast Cancer Res Treat 102:283–288
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, Liedtke C, von Minckwitz G, Nekljudova V et al (2013) Sentinel lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14:609e18
Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB (1996) Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol 9:893e900
Sugie T, Sawada T, Tagaya N, Kinoshita T, Yamagami K, Suwa H, Ikeda T, Yoshimura K, Niimi M, Shimizu A et al (2213e) Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol 20:2213e8
Diego EJ, McAuliffe PF, Soran A, McGuire KP, Johnson RR, Bonaventura M, Ahrendt GM (2016) Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol 23(5):1549–1553
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, Bedrosian I, Hobbs BP, DeSnyder SM, Hwang RF et al (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34(10):1072–1078
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Kuerer HM, Bowling M, Flippo-Morton TS et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310(14):1455–1461
Barthelmes L, Goyal A, Newcombe RG, Mansel RE, NEW START, and ALMANAC study groups (2010) Adverse reactions to patent blue V dye-The NEW START and ALMANAC experience. Eur J Surg Oncol 36:399–403
Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P (2009) Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 96:1295–1299
Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, Jones PA (2011) Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. Am J Roentgenol 196:251–256
Cox K, Sever A, Jones S, Weeks J, Mills P, Devalia H, Fish D, Jones P (2013) Valid-ation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patie-nts with a normal grey-scale axillary ultrasound. Eur J Surg Oncol 39:760–765
Sever AR, Mills P, Hyvelin JM, Weeks J, Gumus H, Fish D, Mali W, Jones SE, Jones PA, Devalia H (2012) Percutaneous removal of sentinel lymph nodes in a swine model using a breast lesion excision system and contrast-enhanced ultrasound. Eur Radiol 22:545–550
Cox K, Taylor-Phillips S, Sharma N, Weeks J, Mills P, Sever A, Lim A, Haigh I, Hashem M, de Silva T et al (2018) Enhanced pre-operative axillary staging using intradermal microbubbles and contrast-enhanced ultrasound to detect and biopsy sentinel lymph nodes in breast cancer: a potential replacement for axillary surgery. Br J Radiol 91:20170626
Nielsen Moody A, Bull J, Culpan AM, Munyombwe T, Sharma N, WhitakerM WS (2017) Preoperative sentinel lymph node identification, biopsy and localisa-tion using contrast enhanced ultrasound (CEUS) in patients with breast cancer: asystematic review and meta-analysis. Clin Radiol 72:959–971
Fu JF, Chen HL, Yang J, Yi CH, Zheng S (2014) Feasibility and accuracy of sentinel lymph node biopsy in clinically node-positive breast cancer after ne-oadjuvant chemotherapy: a meta-analysis. PLoS ONE 9:e105316
Kang YJ, Han W, Park S, You JY, Yi HW, Park S, Nam S, Kim JH, Yun KW, Kim HJ et al (2017) Outcome following sentinel lymph node biopsy-guided decisions in breast cancer patientswith conversion from positive to negative axillary lymph nodes after neoadjuva-nt chemotherapy. Breast Cancer Res Treat 166:473–480
Kim JY, Kim MK, Lee JE, Jung Y, Bae SY, Lee SK, Kil WH, Kim SW, Kim KS, Nam SJ et al (2015) Sentinel lymph node biopsy alone after neoadjuvant chemotherapy in patients with initi-al cytology-proven axillary node metastasis. J Breast Cancer 18:22–28
Newman EA, Sabel MS, Nees AV, Schott A, Diehl KM, Cimmino VM, Chang AE, Kleer C, Hayes DF, Newman LA (2007) Sentinel lymph node biopsy performed after neoadjuvant chemothera-py is accurate in patients with documented node-positive breast cancer at prese-ntation. Ann Surg Oncol 14:2946–2952
Tsuyuki S, Yamaguchi A, Kawata Y, Kawaguchi K (2015) Assessing the effects of neoadjuvant chemotherapy on lymphatic pathways to sentinel lymph nodes in cases of breast cancer: usefulness of the indocyanine green-fluorescence method. Breast 24(3):298–301
Wang Y, Zhou W, Li C, Gong H, Li C, Yang N, Zha X, Chen L, Xia T, Liu X et al (2017) Variation ofsentinel lymphatic channels (SLCs) and sentinel lymph nodes (SLNs) assessed by contrast-enhanced ultrasound (CEUS) in breastcancer patients. World J Surg Oncol 15:127
Breslin TM, Cohen L, Sahin A, Fleming JB, Kuerer HM, Newman LA, Delpassand ES, House R, Ames FC, Feig BW et al (2000) Sentinel lymph node biopsy is accurate after neoadjuvant chemotherapy for breast cancer. J Clin Oncol 18(20):3480–3486
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A et al (1998) Effect of preoperative chemotherapy on the outcome of women with oper-able breast cancer. J Clin Oncol 16(8):2672–2685
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 30:96–102
Nathanson SD, Burke M, Slater R, Kapke A (2007) Preoperative identificati-on of the sentinel lymph node in breast cancer. Ann Surg Oncol 14:3102–3110
Caudle AS, Yang WT, Mittendorf EA, Black DM, Hwang R, Hobbs B, Hunt KK, Krishnamurthy S, Kuerer HM (2015) Selective surgical localization of axillary lymph nodes containing metastases inpatients with breast cancer: a prospective feasibility trial. JAMA Surg 150:137–143
Funding
This work was sponsored by the Natural Science Foundation of Fujian Province (2018J01264), Fujian provincial health technology project (2017-CXB-2), and Joint Funds for the innovation of science and Technology, Fujian province (2017Y9076, 2018Y9113), and Science and Technology Program of Fujian Province(2018Y2003), Fujian provincial health and family planning research talent training program (Grant number: 2016-ZQN-17).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: XW. Performed the experiments: LT, WH, DH, WP, SH, XW. Analyzed the data: XW, LT. Contributed reagents/materials/analysis tools: XW, WP, DH, SH. Wrote the paper: XW.
Corresponding author
Ethics declarations
Conflict of interests
None declared.
Patient consent for publication
Not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
This study was approved by Institutional Review Board of the Affiliated Cancer Hospital of Fujian Medical University, Fujian Provincial Cancer Hospital.
Provenance and peer review
Not commissioned; externally peer reviewed.
Data sharing statement
Data are available upon reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, X., Tang, L., Huang, W. et al. Contrast-enhanced ultrasonography and blue dye methods in detection of sentinel lymph nodes following neoadjuvant chemotherapy in initially node positive breast cancer. Arch Gynecol Obstet 302, 685–692 (2020). https://doi.org/10.1007/s00404-020-05646-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05646-8