Abstract
Purpose
Ovarian tissue (OT) cryopreservation is a treatment option for fertility preservation among young cancer patients. However, the procedure may involve a reduction in the GDF9-β expression and a delay in follicular growth after thawing and transplantation. The aim of this study was to evaluate whether supplementation of GDF9-β can compensate the reduction of this factor during the cryopresevation process and promote folliculogenesis after transplantation of thawed sheep ovarian tissue.
Methods
Sheep OT was cryopreserved using two methods of vitrification and slow freezing. Fresh and thawed OTs were then transplanted onto chick embryo chorioallantoic membrane (CAM) and then divided into two groups based on the addition of GDF9-β to the grafted tissue. After 5 days of culture, both histological and immunohistological (Ki-67) assessments were performed to evaluate follicular structure, development, and proliferation. The fibrotic and necrotic areas were measured using MICROVISIBLE software.
Results
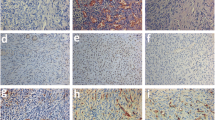
Folliculogenesis took place in all culture groups, but was significantly improved only in the +GDF9-β cultured group. Also, better follicular structure was preserved in the aforementioned group (p < 0.05). When GDF9-β was supplemented to the culture medium, more neovascularization (p < 0.05) and better transplantation (p > 0.05) was observed. Furthermore, the areas of fibrosis and necrosis were lower in this group rather than the controls. Follicular proliferative activity was significantly higher only in the slow freezing +GDF9-β cultured group.
Conclusions
GDF9-β, as a stimulatory factor, not only promoted the folliculogenesis in the fresh ovarian transplant, but also compensated for its reduction during the cryopreservation process.





Similar content being viewed by others
References
Langbeen A, Bartholomeus E, Leroy JL, Bols PE (2015) Bovine in vitro reproduction models can contribute to the development of (female) fertility preservation strategies. Theriogenology 84(4):477–489
Youm HW, Lee JR, Lee J, Jee BC, Suh CS, Kim SH (2015) Transplantation of mouse ovarian tissue: comparison of the transplantation sites. Theriogenology 83(5):854–861
Lee D (2007) Ovarian tissue cryopreservation and transplantation: banking reproductive potential for the future. Oncofertility fertility preservation for cancer survivors. Springer, pp 110–129
Tanpradit N, Comizzoli P, Srisuwatanasagul S, Chatdarong K (2015) Positive impact of sucrose supplementation during slow freezing of cat ovarian tissues on cellular viability, follicle morphology, and DNA integrity. Theriogenology 83(9):1553–1561
Demirci B, Lornage J, Salle B, Poirel MT, Guerin JF, Franck M (2003) The cryopreservation of ovarian tissue: uses and indications in veterinary medicine. Theriogenology 60(6):999–1010
Jorssen EP, Langbeen A, Fransen E, Martinez EL, Leroy JL, Bols PE (2014) Monitoring preantral follicle survival and growth in bovine ovarian biopsies by repeated use of neutral red and cultured in vitro under low and high oxygen tension. Theriogenology 82(3):387–395
Fatehi R, Ebrahimi B, Shahhosseini M, Farrokhi A, Fathi R (2014) Effect of ovarian tissue vitrification method on mice preantral follicular development and gene expression. Theriogenology 81(2):302–308
Telfer EE, Zelinski MB (2013) Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 99(6):1523–1533
Isachenko V, Mallmann P, Petrunkina AM, Rahimi G, Nawroth F, Hancke K, Felberbaum R, Genze F, Damjanoski I, Isachenko E (2012) Comparison of in vitro-and chorioallantoic membrane (CAM)-culture systems for cryopreserved medulla-contained human ovarian tissue. PLoS One 7(3):e32549
Martinez-Madrid B, Donnez J, Van Eyck A-S, Veiga-Lopez A, Dolmans M-M, Van Langendonckt A (2009) Chick embryo chorioallantoic membrane (CAM) model: a useful tool to study short-term transplantation of cryopreserved human ovarian tissue. Fertil Steril 91(1):285–292
Qureshi A, Nussey S, Bano G, Musonda P, Whitehead S, Mason H (2008) Testosterone selectively increases primary follicles in ovarian cortex grafted onto embryonic chick membranes: relevance to polycystic ovaries. Reproduction 136(2):187–194
Isachenko V, Orth I, Isachenko E, Mallmann P, Peters D, Schmidt T, Morgenstern B, Foth D, Hanstein B, Rahimi G (2013) Viability of human ovarian tissue confirmed 5 years after freezing with spontaneous ice-formation by autografting and chorio-allantoic membrane culture. Cryobiology 66(3):233–238
Gigli I, Cushman R, Wahl C, Fortune J (2005) Evidence for a role for anti-Müllerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev 71(4):480–488
Hunter M, Robinson R, Mann G, Webb R (2004) Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim Reprod Sci 82:461–477
Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppä L, Louhio H, Tuuri T, Sjöberg J, Bützow R (1999) Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab 84(8):2744–2750
Cushman R, Wahl C, Fortune J (2002) Bovine ovarian cortical pieces grafted to chick embryonic membranes: a model for studies on the activation of primordial follicles. Hum Reprod 17(1):48–54
Celestino J, Lima-Verde I, Bruno J, Matos M, Chaves R, Saraiva M, Silva C, Faustino L, Rossetto R, Lopes C (2011) Steady-state level of bone morphogenetic protein-15 in goat ovaries and its influence on in vitro development and survival of preantral follicles. Mol Cell Endocrinol 338(1):1–9
Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S (2014) The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update 20(6):869–883
Fenwick MA, Mora JM, Mansour YT, Baithun C, Franks S, Hardy K (2013) Investigations of TGF-β signaling in preantral follicles of female mice reveal differential roles for bone morphogenetic protein 15. Endocrinology 154(9):3423–3436
Vilela J, Leonel E, D’Oliveira L, Paiva R, Miranda-Vilela A, Amorim C, Pic-Taylor A, Lucci C (2016) Culture of domestic cat ovarian tissue in vitro and in the chick embryo chorioallantoic membrane. Theriogenology 86(7):1774–1781
Aerts JM, De Clercq JB, Andries S, Leroy JL, Van Aelst S, Bols PE (2008) Follicle survival and growth to antral stages in short-term murine ovarian cortical transplants after cryologic solid surface vitrification or slow-rate freezing. Cryobiology 57(2):163–169
Mofarahe ZS, Salehnia M, Novin MG, Ghorbanmehr N, Fesharaki MG (2017) Expression of folliculogenesis-related genes in vitrified human ovarian tissue after two weeks in vitro culture. Cell J (Yakhteh) 19(1):18
Chakravarthi VP, Kona S, Kumar AS, Bhaskar M, Rao V (2015) Quantitative expression of antiapoptotic and proapoptotic genes in sheep ovarian follicles grown in vivo or cultured in vitro. Theriogenology 83(4):590–595
Aguiar F, Lunardi F, Lima L, Rocha R, Bruno J, Magalhães-Padilha D, Cibin F, Rodrigues A, Gastal M, Gastal E (2016) Insulin improves in vitro survival of equine preantral follicles enclosed in ovarian tissue and reduces reactive oxygen species production after culture. Theriogenology 85(6):1063–1069
Behrouzi A, Colazo MG, Ambrose DJ (2016) Alterations in bone morphogenetic protein 15, growth differentiation factor 9, and gene expression in granulosa cells in preovulatory follicles of dairy cows given porcine LH. Theriogenology 85(7):1249–1257
Thomas FH, Vanderhyden BC (2006) Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol 4(1):19
Kagawa N, Silber S, Kuwayama M (2009) Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online 18(4):568–577
Wang Y, Xiao Z, Li L, Fan W, Li S-W (2008) Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod 23(10):2256–2265
Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, Bader M, Weiss J (2009) Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction 138(2):319–327
Martins F, Celestino J, Saraiva M, Matos M, Bruno J, Rocha-Junior C, Lima-Verde I, Lucci C, Báo S, Figueiredo J (2008) Growth and differentiation factor-9 stimulates activation of goat primordial follicles in vitro and their progression to secondary follicles. Reprod Fertil Dev 20(8):916–924
Author information
Authors and Affiliations
Contributions
MV and MM: protocol/project development. MV and NY: data collection or management. NY: data analysis. BW: manuscript writing/editing. MAK: manuscript writing/editing
Corresponding author
Ethics declarations
Conflict of interest
The research did not get any financial support from any funding agency regarding the material. No conflicts of interest with the authors to be declared.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The use of slaughterhouse derived ovaries and the CAM culture system does not raise ethical or legal concerns, nor does it violate the animal protection laws [9].
Rights and permissions
About this article
Cite this article
Vatanparast, M., Khalili, M.A., Yari, N. et al. GDF9-β promotes folliculogenesis in sheep ovarian transplantation onto the chick embryo chorioallantoic membrane (CAM) in cryopreservation programs. Arch Gynecol Obstet 298, 607–615 (2018). https://doi.org/10.1007/s00404-018-4838-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4838-x