Abstract
Purpose
Human papillomaviruses (HPV) have been associated with placental inflammation resulting in high-risk preterm birth. The premise for this study was that treatment with anti-inflammatory pentoxifylline would inhibit HPV-mediated placental cell death. The objectives were: (1) to study the effects of HPV-16 exposure on trophoblast cells and (2) to evaluate pentoxifylline in preventing the damaging effects of HPV-16.
Methods
Mouse embryos (1-cell) were cultured (G1plus medium, 72 h, 37 °C, 5 % CO2 in air), divided into four groups at blastocyst-stage and incubated to implantation stage. Implanted trophoblasts were exposed to either HPV-16 (group 1), concomitant HPV-16 and 3 mM pentoxifylline (group 2), 3 mM pentoxifylline only (group 3) or control medium (group 4) and further incubated for 24 h. HPV-16 were from SiHa cell lysates. Trophoblast structural integrity was assessed by morphometric analysis while apoptosis and necrosis were detected using dual-stain fluorescence assay.
Results
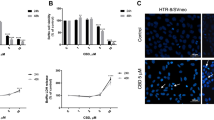
Trophoblast outgrowth was reduced by 90 % (P < 0.05) in HPV-16 presence (629 ± 265 µ2, mean + SEM) when compared with controls (6,456 ± 795 µ2). Pentoxifylline attenuated the effects of HPV-16 (4,308 ± 362 µ2). Nuclear size of HPV-16 infected trophoblasts was smallest among the groups (P < 0.005). HPV-16 decreased cell viability and increased necrosis but not apoptosis. Pentoxifylline prevented HPV-16 associated necrosis in trophoblasts.
Conclusions
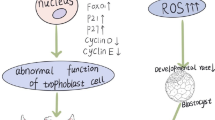
HPV-16 decreased nuclear size and trophoblast outgrowth but these effects were attenuated by the phosphodiesterase inhibitor, pentoxifylline. The action of HPV-16 on trophoblasts was increased cell necrosis suggesting that HPV-16 pathogenesis involved either cAMP inhibition and/or activated TNF pathways.

Similar content being viewed by others
References
Munoz N, Castellsagué X, de Gonzalez AB, Gissmann L (2006) HPV in the etiology of human cancer. Vaccine 24:1–10
Syrjänen K, Syrjänen S (1990) Epidemiology of human papilloma virus infections and genital neoplasia. Scand J Infect Dis Suppl 69:7–17 (Review)
Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE (2007) Prevalence of HPV infection among females in the United States. JAMA 297:813–819
Weyn C, Thomas D, Jani J, Guizani M, Donner C, Van Rysselberge M, Hans C, Bossens MYE, Fontaine V (2011) Evidence for human papillomavirus in the placenta. J Infect Dis 203:341–343
Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S (2008) Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod 23:709–715
Sarkola ME, Grénman SE, Rintala MA, Syrjnen KJ, Syrjnen SM (2008) Human papillomavirus in the placenta and umbilical cord blood. Acta Obstet Gynecol Scand 87:1181–1188
You H, Liu Y, Agrawal N, Prasad CK, Edwards JL, Osborne AF, Korourian S, Lowery CL, Hermonat PL (2008) Multiple human papillomavirus types replicate in 3A trophoblasts. Placenta 29:30–38
Manavi M, Czerwenka KF, Schurz B, Knogler W, Kubista E, Reinold E (1992) Latent cervical virus infection as a possible cause of early abortion. Gynakol Geburtshilfliche Rundsch 32:84–87
Hermonat PL, Han L, Wendel PJ, Quirk JG, Stern S, Lowery CL, Rechtin TM (1997) Human papillomavirus is more prevalent in first trimester spontaneously aborted products of conception compared to elective specimens. Virus Genes 14:13–17
Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinnery M, Lasn J, Born Too Soon Preterm Birth Action Group (2013) Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 10 Suppl 1:S2
Vrachnis N, Vitoratos N, Iliodromiti Z, Sifakis S, Deligeoroglou E, Creatsas G (2010) Intrauterine inflammation and preterm delivery. Ann N Y Acad Sci 1205:118–122
Zuo Z, Goel S, Carter JE (2011) Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm birth. Am J Clin Pathol 135:260–265
Cho G, Min KJ, Hong HR, Kim S, Hong JH, Lee JK, Oh MJ, Kim H (2013) High-risk human papillomavirus infection is associated with premature rupture of membranes. BMC Pregnancy Childbirth 6:173. doi:10.1186/1471-2393-13-173
McDonnold M, Dunn H, Hester A, Pacheco LD, Hankins GD, Saade GR, Costantine MM (2014) High risk human papillomavirus at entry to prenatal care and risk of preeclampsia. Am J Obstet Gynecol 210:138.e1–138.e5
Harris E, Schulzke SM, Patole SK (2010) Pentoxifylline in preterm neonates: a systematic review. Paediatr Drugs 12:301–311
Lauterbach R, Rytlewski K, Pawlik D, Hurkała J, Wójtowicz A, Bręborowicz G, Szymankiewicz M (2012) Effect of pentoxifylline, administered in preterm labour, on the foetal-placental circulation and neonatal outcome: a randomized, prospective pilot study. Basic Clin Pharmacol Toxicol 110:342–346
Hong LJ, Oshiro BT, Chan PJ (2013) HPV-16 exposed mouse embryos: a potential model for pregnancy wastage. Arch Gynecol Obstet 287:1093–1097
Meissner JD (1999) Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J Gen Virol 80:1725–1733
Tungteakkhun SS, Filippova M, Neidigh JW, Fodor N, Duerksen-Hughes PJ (2008) The interaction between human papillomavirus type 16 and FADD is mediated by a novel E6 binding domain. J Virol 82:9600–9614
Rowland SC, Jacobson JD, Patton WC, King A, Chan PJ (2003) Dual fluorescence analysis of DNA apoptosis in sperm. Am J Obstet Gynecol 188:1156–1157
Chan PJ, Corselli JU, Jacobson JD, Patton WC, King A (1999) Spermac stain analysis of human sperm acrosomes. Fertil Steril 72:124–128
Han J, Li L, Hu J, Yu L, Zheng Y, Guo J, Zheng X, Yi P, Zhou Y (2010) Epidermal growth factor stimulates human trophoblast cell migration through Rho A and Rho C activation. Endocrinology 151:1732–1742
Hinck L, Thissen JP, Pampfer S, De Hertogh R (2003) Effect of high concentrations of glucose on differentiation of rat trophoblast cells in vitro. Diabetologia 46:276–283
Mazurek AM, Fiszer-Kierzkowska A, Rutkowski T, Składowski K, Pierzyna M, Scieglińska D, Woźniak G, Głowacki G, Kawczyński R, Małusecka E (2013) Optimization of circulating cell-free DNA recovery for KRAS mutation and HPV detection in plasma. Cancer Biomark 13:385–394
Wang J, Mayernik L, Armant DR (2007) Trophoblast adhesion of the peri-implantation mouse blastocyst is regulated by integrin signaling that targets phospholipase C. Dev Biol 302:143–153
Boulenouar S, Weyn C, Van Noppen M, Moussa Ali M, Favre M, Delvenne PO, Bex F, Noël A, Englert Y, Fontaine V (2010) Effects of HPV-16 E5, E6 and E7 proteins on survival, adhesion, migration and invasion of trophoblastic cells. Carcinogenesis 31:473–480
You H, Liu Y, Carey MJ, Lowery CL, Hermonat PL (2002) Defective 3A trophoblast-endometrial cell adhesion and altered 3A growth and survival by human papillomavirus type 16 oncogenes. Mol Cancer Res 1:25–31
You H, Liu Y, Agrawal N, Prasad CK, Chiriva-Internati M, Lowery CL, Kay HH, Hermonat PL (2003) Infection, replication, and cytopathology of human papillomavirus type 31 in trophoblasts. Virology 316:281–289
Whiteside MA, Siegel EM, Unger ER (2008) Human papillomavirus and molecular considerations for cancer risk. Cancer 113:2981–2994
Terra AP, Murta EF, Maluf PJ, Caballero OL, Brait M, Adad SJ (2007) Aberrant promoter methylation can be useful as a marker of recurrent disease in patients with cervical intraepithelial neoplasia grade III. Tumori 93:572–579
Long BR, Erickson AE, Chapman JM, Barbour JD, Vu BA, Ho EL, Lanier LL, Sauer MM, Carvalho KI, Nixon DF, Kallas EG (2010) Increased number and function of natural killer cells in human immunodeficiency virus 1-positive subjects co-infected with herpes simplex virus 2. Immunology 129:186–196
Li AS, Siu MK, Zhang H, Wong ES, Chan KY, Ngan HY, Cheung AN (2008) Hypermethylation of SOX2 gene in hydatidiform mole and choriocarcinoma. Reprod Sci 15:735–744
Basile JR, Zacny V, Munger K (2001) The cytokines tumor necrosis factor-alpha (TNF-alpha) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J Biol Chem 276:22522–22528
Jin L, Sturgis EM, Zhang Y, Huang Z, Song X, Li C, Wei Q, Li G (2013) Association of tumor necrosis factor-alpha promoter variants with risk of HPV-associated oral squamous cell carcinoma. Mol Cancer 12:80. doi:10.1186/1476-4598-12-80
Sorimachi K, Waring P, Hapel AJ, Fukasawa I, Kaneko Y, Masawa N (2013) Characterisation of apoptosis in myb-transformed hematopoietic cell (MTHC-A) lines: TNF-α-induced apoptosis and prevention by cAMP. J Clin Exp Hematop 53:115–120
Desaintes C, Goyat S, Garbay S, Yaniv M, Thierry F (1999) Papillomavirus E2 induces p53-independent apoptosis in HeLa cells. Oncogene 18:4538–4545
Mighty KK, Laimins LA (2014) The role of human papillomaviruses in oncogenesis. Recent Results Cancer Res 193:135–148 (Review)
Acknowledgments
The SiHa cells were kindly donated by Drs. Duerksen-Hughes and her laboratory team, the Department of Basic Sciences in the Division of Biochemistry at Loma Linda University, CA, USA.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S.S., Block, B.S. & Chan, P.J. Pentoxifylline attenuates HPV-16 associated necrosis in placental trophoblasts. Arch Gynecol Obstet 291, 647–652 (2015). https://doi.org/10.1007/s00404-014-3471-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3471-6