Abstract
Purpose
We present a systematic review to evaluate failure rates (secondary hysterectomy or maternal mortality) and success rates (subsequent menstruation or pregnancy) after different uterus preserving treatment modalities in women with invasive placentation.
Methods
A review of English, German or Dutch language-published research, using Medline and Embase databases, was performed. Studies of any design were included.
Results
Ten cohort studies and 50 case series or case reports were included. Expectant management reported a secondary hysterectomy in 55/287 (19%), maternal mortality in 1/295 (0.3%), a subsequent menstruation in 44/49 (90%) and a subsequent pregnancy in 24/36 (67%). Embolization of the uterine arteries described a secondary hysterectomy in 8/45 (18%), a subsequent menstruation in 8/13 (62%) and a subsequent pregnancy in 5/33 (15%). Methotrexate therapy presented a secondary hysterectomy in 1/16 (6%), a subsequent menstruation in 4/5 (80%) and a subsequent pregnancy in 1/2 (50%). Uterus preserving surgery showed a secondary hysterectomy in 24/77 (31%), maternal mortality in 2/55 (4%), a subsequent menstruation in 28/34 (82%) and a subsequent pregnancy in 19/26 (73%).
Conclusions
This review indicates that different uterus preserving treatment modalities may be effective in managing invasive placentation. Despite the extensive review of the literature, no conclusions about the superiority of any modality can be drawn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Placental implantation in which there is abnormally firm adherence to the uterine wall is defined as placenta increta as well as related conditions like placenta accreta and percreta [1]. This is a challenging obstetrical problem causing severe maternal morbidity like uterine perforation, infection and severe hemorrhage. Severe bleeding is the single most significant cause of maternal death worldwide [2]. Invasive placentation affects ~2% of all singleton deliveries [3]. Probably due to the increasing rates of caesarean deliveries in most countries, the incidence has increased in recent years [4]. Because previous studies reported better maternal survival with hysterectomy than with uterus preserving treatment modalities, a hysterectomy has long been the initial therapy [2]. However, preserving uterine function is important to preserve reproductive potential.
Several case reports indicate that uterus preserving treatment may result in successful management of invasive placentation. In the current literature, different uterus preserving treatment modalities are described: expectant management, embolization of the uterine arteries, methotrexate therapy and uterus preserving surgery [2, 5, 6]. In 2007, Timmermans et al. [6] reviewed 48 case reports about the obstetric outcome after expectant management, embolization of the uterine arteries and methotrexate therapy for invasive placentation. They concluded that it should only be considered in highly selected cases and that no proof was found for a first choice uterus preserving treatment modality.
We present a literature review to evaluate failure rates (secondary hysterectomy or maternal mortality) and success rates (subsequent menstruation or pregnancy) after different uterus preserving treatment modalities in women with invasive placentation.
Methods
Search strategy
A computer-aided search of Medline and Embase was carried out. The following search terms were used: ‘placenta accreta’, ‘placenta increta’, ‘placenta percreta’ and ‘conservative treatment’ (Appendix 1). The reference lists of identified studies were searched for additional relevant studies.
Inclusion criteria
Every study design that was published in English, German or Dutch was considered for inclusion. Given that randomized-controlled trials and large observational cohort studies that can be used to define best practice are lacking, studies of any design were obtained for further evaluation. Studies were included if they described the course of uterus preserving treatment modalities for patients with placenta accreta, increta or percreta. Uterus preserving treatment modalities were defined as initial therapy consisting of: expectant management (expectant management for patients who delivered vaginally or closing the hysterectomy as caesarean delivery occurred), embolization of the uterine arteries, methotrexate therapy or uterus preserving surgery. Because we investigated uterus preserving techniques in which the placenta was left in situ, we limited uterus preserving surgery to hemostatic sutures, arterial ligation and balloon tamponade. Diagnoses of invasive placentation must be made upon clinical suspicion, ultrasound or magnetic resonance imaging (MRI). Studies were excluded if patients underwent a hysterectomy as initial management, or if patients were approached conservatively because caesarean hysterectomy was considered too dangerous or difficult.
Selection of studies
The first reviewer (CN) screened the titles and abstracts of identified articles for eligibility. Papers that seemed to be relevant were obtained, and the full text articles were screened for inclusion. If there was doubt about the suitability of the studies, they were discussed with two other independent reviewers (TP, TE).
Data extraction and analysis
The eligible articles were summarized in a data extraction form, including the following items: obstetric characteristics, maternal morbidity/mortality and subsequent pregnancy/menstruation. Obstetric characteristics included gestational age and mode of delivery. Maternal morbidity/mortality was defined as severe vaginal bleeding (need for blood transfusion or >1,000 ml blood loss), sepsis (definition used according to the definitions of the authors in the different studies), a secondary hysterectomy or maternal mortality.
Data were presented as numbers and as percentages (rates). Rates were calculated using the reported number of a specific item as the numerator divided by all studies that reported that specific item as denominator.
Data were summarized separately for expectant management, methotrexate therapy, embolization of the uterine arteries and uterus preserving surgery with distinction between forms of invasive placentation (placenta accreta, increta or percreta).
Data extraction and analysis was done by the first reviewer (CN).
Results
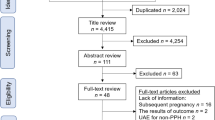
We identified 1,477 articles, of which 270 were potentially relevant after removing duplicates and screening the title and abstract. Applying our inclusion criteria led to the inclusion of 10 cohort studies and 50 case series or case reports describing 434 patients. Of them, 295 patients treated with expectant management, 45 with embolization of the uterine arteries, 17 with methotrexate therapy and 77 with uterus preserving surgery were reported (Fig. 1).
Expectant management (Table 1)
Twenty articles described 295 patients with invasive placentation approached with expectant management [7–25]: a secondary hysterectomy occurred in 55/287 (19%), maternal mortality in 1/295 (0.3%), a subsequent menstruation in 44/49 (90%) and a subsequent pregnancy in 24/36 (67%).
Embolization of the uterine arteries (Table 2)
Sixteen articles reported 45 patients managed with embolization of the uterine arteries [12, 26–40]: a secondary hysterectomy occurred in 8/45 (18%), a subsequent menstruation in 8/13 (62%) and a subsequent pregnancy in 5/33 (15%). All patients survived until the end of follow up.
Methotrexate therapy (Table 3)
Fifteen articles showed 17 patients receiving methotrexate therapy [21, 41–54]: a secondary hysterectomy occurred in 1/16 (6%), a subsequent menstruation in 4/5 (80%) and a subsequent pregnancy in 1/2 (50%). All patients from the studies survived until the end of follow up.
Uterus preserving surgery (Table 4)
Eleven articles presented 77 patients with uterus preserving surgery [55–65]: a secondary hysterectomy occurred in 24/77 (31%), maternal mortality in 2/55 (4%), a subsequent menstruation in 28/34 (82%) and a subsequent pregnancy in 19/26 (73%).
Comment
The aim of the current review was to evaluate failure and success rates of women with invasive placentation managed with different uterus preserving treatment modalities. The most important gain of uterus preserving treatment is preserving reproductive material. Our results show varying failure and success rates among the different uterus preserving treatment modalities: a secondary hysterectomy was needed in 6–31% and maternal mortality occurred in 0–4% (failure rates); menstruation followed in 62–90% and a subsequent pregnancy occurred in 15–73% (success rates). Our results are based on descriptive data only (case series, case reports and a few cohort studies). Therefore, it is not possible to compare different uterus preserving treatment modalities and no conclusions about the superiority of any modality can be drawn.
Uterus preserving treatment modalities in general
Given the risk of substantial morbidity (including coagulopathy, severe hemorrhage, infection, sepsis, ureteral injury, need for blood transfusion/hysterectomy) and mortality, uterus preserving treatment may have a role in carefully selected patients who desire future fertility [66]. The patient should be counselled about the risk of hysterectomy, blood transfusion and even death. Prophylactic antibiotics are generally administrated to prevent infection [18]. When conservative management is successful, it results in gradual resorption of the placenta or delayed delivery of the placenta [15, 17, 28]. Due to the risk of severe hemorrhage, all obstetric units and practitioners must have the facilities, personnel, and equipment in place to manage this emergency properly and a multidisciplinary approach is recommended [2, 67].
Expectant management
Whether adjuvant therapy in addition to expectant management alone is beneficial is uncertain. Timmermans et al. [6] reported 60 cases with abnormally invasive placentation. Expectant management was successful in 48 cases, but adjuvant therapy (uterine arterial embolization and methotrexate therapy) was employed in 34 cases.
Uterine arterial embolization
Arterial embolization is a viable treatment for postpartum bleeding. A patient with stable vital sings and persistent bleeding, especially if the rate of loss is not excessive, may be a candidate for arterial embolization [2]. A previous Cochrane review [68] compared uterine arterial embolization with hysterectomy for symptomatic uterine fibroids. They concluded that uterine arterial embolization offers an advantage over hysterectomy with regard to a shorter hospital stay and a quicker return to routine activities. Specific complications from this procedure include iliac artery thrombosis, uterine necrosis or sepsis resulting in multiple organ failure. In addition, non-target embolization can cause ischaemic damage to other organs [69].
Methotrexate therapy
Methotrexate disrupts the folic acid pathway in rapidly dividing cells such as trophoblasts. However, the proliferation of trophoblasts in the later stages of pregnancy has been shown to have no role in placental growth [66]. Consequently, the use of methotrexate may not reduce placental volume. This therapy might even be harmful: methotrexate has a immunosuppressive role and therefore could increase the risk of infection or even sepsis, which is already increased in patients with abnormal adherent placentation. Other specific adverse effects are methotrexate-related pancytopenia and nephrotoxicity [70, 71].
Uterus preserving surgery
Uterine compression sutures function in a manner similar to manual compression and are placed to prevent uterine relaxation due to the retained placenta [72]. Arterial occlusion (arterial ligation or balloon tamponade) is indicated for the management of bleeding. In some cases, a combination of both techniques was used [58]. Because we investigated uterus preserving techniques in which the placenta was left in situ, we limited uterus preserving surgery to hemostatic sutures, arterial ligation and balloon tamponade. Other surgical techniques focussing on resection of the invasive placentation are described [73]. In addition to technical advances in vascular control and tissue repair, these surgical resection techniques may contribute to future better uterus preserving surgical options.
Limitations
Due to the descriptive data, this review has a narrative character. The biggest limitation of descriptive data gathered from published case reports and series is that these data are subject to publication bias. The data may be misleading, giving uterus preserving treatment modalities a higher than true success rate. People tend to write up case reports of cases they did that went well; they are less likely to write up the case report about the patients who died or had major complications from uterus preserving treatment. Severe complications are prone to being underreported.
In addition, the cases are limited by the ability to fully determine correct documentation of correct pregnancy or long-term outcome/complications of these pregnancies. In most case reports, data are lacking, which make it difficult to draw conclusions.
Furthermore, categorizing each case specifically based upon the type of uterus preserving treatment modality is difficult since there are varying degrees of placental attachment abnormalities and varying amounts of the placenta which adhere abnormally to the uterus. The uterine preserving treatment modality is a surgical decision based upon particular characteristics of the problem and the expertise of the surgeon. The choice of uterus preserving treatment modality is intricately linked with the degree of placental volume involved. Specific uterus preserving treatment modalities may have the best outcome because the volume of placental involvement is less. Bad outcomes may be employed in large volume placental involvement. The results may simply be a function of disease severity.
However, evaluation of uterus preserving treatment is important and of great clinical use because of the possibility of a subsequent pregnancy. Large-scale studies are required using prospective and repeated measure designs to further evaluate the safety, efficacy and fertility effects.
Conclusion
This review indicates that different uterus preserving treatment modalities may be effective in managing invasive placentation. Despite the extensive review of the literature, no conclusions about the superiority of any modality can be drawn.
References
Irving FC, Hertig AT (1937) A study of placenta accrete. Surg Gynaecol Obstet 64:178–200
ACOG Committee on Obstetric Practice (2002) ACOG Committee opinion. Number 266, January 2002: placenta accreta. Obstet Gynecol 99(1):169–170
Dombrowski MP, Bottoms SF, Saleh AA, Hurd WW, Romero R (1995) Third stage of labor: analysis of duration and clinical practice. Am J Obstet Gynecol 172(4 Pt 1):1279–1284
Miller DA, Chollet JA, Goodwin TM (1997) Clinical risk factors for placenta previa–placenta accreta. Am J Obstet Gynecol 177(1):210–214
Alanis M, Hurst BS, Marshburn PB, Matthews ML (2006) Conservative management of placenta increta with selective arterial embolization preserves future fertility and results in a favorable outcome in subsequent pregnancies. Fertil Steril 86(5):1514.e3– 1514.e7
Timmermans S, van Hof AC, Duvekot JJ (2007) Conservative management of abnormally invasive placentation. Obstet Gynecol Surv 62(8):529–539
Davis JD, Cruz A (1996) Persistent placenta increta: a complication of conservative management of presumed placenta accreta. Obstet Gynecol 88(4 Pt 2):653–654
Bennett MJ, Townsend L (2009) Conservative management of clinically diagnosed placenta accreta following vaginal delivery. Aust N Z J Obstet Gynaecol 49(6):647–649
Chiang YC, Shih JC, Lee CN (2006) Septic shock after conservative management for placenta accreta. Taiwan J Obstet Gynecol 45(1):64–66
Jwarah E, Wilkin DJ (2006) Conservative management of placenta accreta. J Obstet Gynaecol 26(4):378–379
Kayem G, Anselem O, Schmitz T, Goffinet F, Davy C, Mignon A et al (2007) Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris) 36(7):680–687
Kayem G, Clement D, Goffinet F (2007) Recurrence following conservative management of placenta accreta. Int J Gynaecol Obstet 99(2):142–143
Komulainen MH, Vayrynen MA, Kauko ML, Saarikoski S (1995) Two cases of placenta accreta managed conservatively. Eur J Obstet Gynecol Reprod Biol 62(1):135–137
Sinha P, Oniya O, Bewley S (2005) Coping with placenta praevia and accreta in a DGH setting and words of caution. J Obstet Gynaecol 25(4):334–338
Matsumura N, Inoue T, Fukuoka M, Sagawa N, Fujii S (2000) Changes in the serum levels of human chorionic gonadotropin and the pulsatility index of uterine arteries during conservative management of retained adherent placenta. J Obstet Gynaecol Res 26(2):81–87
Taylor AA, Sanusi FA, Riddle AF (2001) Expectant management of placenta accreta following stillbirth at term: a case report. Eur J Obstet Gynecol Reprod Biol 96(2):220–222
Hatfield JL, Brumsted JR, Cooper BC (2006) Conservative treatment of placenta accreta. J Minim Invasive Gynecol 13(6):510–513
Sentilhes L, Ambroselli C, Kayem G, Provansal M, Fernandez H, Perrotin F et al (2010) Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol 115(3):526–534
Bretelle F, Courbière B, Mazouni C, Agostini A, Cravello L, Boubli L, Gamerre M, D'Ercole C (2007) Management of placenta accreta: morbidity and outcome. Eur J Obstet Gynecol Reprod Biol 133(1):34–39
Provansal M, Courbiere B, Agostini A, D’Ercole C, Boubli L, Bretelle F (2010) Fertility and obstetric outcome after conservative management of placenta accreta. Int J Gynaecol Obstet 109(2):147–150
Panoskaltsis TA, Ascarelli A, de Souza N, Sims CD, Edmonds KD (2000) Placenta increta: evaluation of radiological investigations and therapeutic options of conservative management. BJOG 107(6):802–806
O’Brien JM, Barton JR, Donaldson ES (1996) The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol 175(6):1632–1638
Lee LC, Lin HH, Wang CW, Cheng WF, Huang SC (1995) Successful conservative management of placenta percreta with rectal involvement in a primigravida. Acta Obstet Gynecol Scand 74(10):839–841
Veenstra MJ, Spinder T, Dekker GA, van Geijn HP (1995) Post partum intra-abdominal hemorrhage due to placenta percreta. Eur J Obstet Gynecol Reprod Biol 62(2):253–256
Teo SB, Kanagalingam D, Tan HK, Tan LK (2008) Massive postpartum haemorrhage after uterus-conserving surgery in placenta percreta: the danger of the partial placenta percreta. BJOG 115(6):789–792
Kayem G, Pannier E, Goffinet F, Grange G, Cabrol D (2002) Fertility after conservative treatment of placenta accreta. Fertil Steril 78(3):637–638
Sivan E, Spira M, Achiron R, Rimon U, Golan G, Mazaki-Tovi S et al (2010) Prophylactic pelvic artery catheterization and embolization in women with placenta accreta: can it prevent cesarean hysterectomy? Am J Perinatol 27(6):455–461
Breathnach F, Tuite DJ, McEniff N, Byrne P, Geary MP (2007) Uterine artery embolisation as an interval adjunct to conservative management of placenta praevia increta. J Obstet Gynaecol 27(2):195
Takeda A, Koyama K, Imoto S, Mori M, Nakano T, Nakamura H (2010) Conservative management of placenta increta after first trimester abortion by transcatheter arterial chemoembolization: a case report and review of the literature. Arch Gynecol Obstet 281(3):381–386
Liao CY, Ding DC (2009) Failure of conservative treatment for placenta increta. Taiwan J Obstet Gynecol 48(3):302–304
Clement D, Kayem G, Cabrol D (2004) Conservative treatment of placenta percreta: a safe alternative. Eur J Obstet Gynecol Reprod Biol 114(1):108–109
Bennett MJ, Sen RC (2003) ‘Conservative’ management of placenta praevia percreta: report of two cases and discussion of current management options. Aust NZJ Obstet Gynaecol 43(3):249–251
Tan CH, Tay KH, Sheah K, Kwek K, Wong K, Tan HK et al (2007) Perioperative endovascular internal iliac artery occlusion balloon placement in management of placenta accreta. Am J Roentgenol 189(5):1158–1163
Diop AN, Bros S, Chabrot P, Gallot D, Boyer L (2009) Placenta percreta: urologic complication after successful conservative management by uterine arterial embolization: a case report. Am J Obstet Gynecol 201(5):e7–e8
Luo G, Perni SC, Jean-Pierre C, Baergen RN, Predanic M (2005) Failure of conservative management of placenta previa–percreta. J Perinat Med 33(6):564–568
Tseng SH, Lin CH, Hwang JI, Chen WC, Ho ES, Chou MM (2006) Experience with conservative strategy of uterine artery embolization in the treatment of placenta percreta in the first trimester of pregnancy. Taiwan J Obstet Gynecol 45(2):150–154
Dinkel HP, Durig P, Schnatterbeck P, Triller J (2003) Percutaneous treatment of placenta percreta using coil embolization. J Endovasc Ther 10(1):158–162
Yee YH, Kung FT, Yu PC, Hsu TY, Cheng YF (2008) Successful conservative management of placenta previa totalis and extensive percreta. Taiwan J Obstet Gynecol 47(4):431–434
Descargues G, Clavier E, Lemercier E, Sibert L (2000) Placenta percreta with bladder invasion managed by arterial embolization and manual removal after cesarean. Obstet Gynecol 96(5 pt 2):840
Butt K, Gagnon A, Delisle MF (2002) Failure of methotrexate and internal iliac balloon catheterization to manage placenta percreta. Obstet Gynecol 99(6):981–982
Morken NH, Kahn JA (2006) Placenta accreta and methotrexate treatment. Acta Obstet Gynecol Scand 85(2):248–250
Cole MD (2002) Using methotrexate to treat placenta accreta. AWHONN Lifelines 6(6):486–487
Crespo R, Lapresta M, Madani B (2005) Conservative treatment of placenta increta with methotrexate. Int J Gynaecol Obstet 91(2):162–163
Wehbe SA, Ghulmiyyah LM, Carroll KT, Perloe M, Schwartzberg DG, Sills ES (2003) Correlations from gadopentetate dimeglumine-enhanced magnetic resonance imaging after methotrexate chemotherapy for hemorrhagic placenta increta. Biomagn Res Technol 1(1):3
Zepiridis L, Zafrakas M, Theodoridis TD, Assimakopoulos E, Tzevelekis P, Athanatos D et al (2009) Human placental lactogen and color Doppler in predicting expulsion of retained adherent placenta: a new clinical observation. Arch Gynecol Obstet 280(6):1041–1044
Adair SR, Elamin D, Tharmaratnam S (2004) Placenta increta; conservative management—a successful outcome. Case report and literature review. J Matern Fetal Neonatal Med 15(4):275–278
Endo T, Hayashi T, Shimizu A, Matsuura M, Mizuuchi M, Nagasawa K et al (2009) Successful uterus-preserving surgery for treatment of chemotherapy-resistant placenta increta. Gynecol Obstet Invest 69(2):112–115
Otsubo Y, Shinagawa T, Chihara H, Araki T (1999) Conservative management of a case of placenta praevia percreta. Aust N Z J Obstet Gynaecol 39(4):518–519
Heiskanen N, Kroger J, Kainulainen S, Heinonen S (2008) Placenta percreta: methotrexate treatment and MRI findings. Am J Perinatol 25(2):91–92
Legro RS, Price FV, Hill LM, Caritis SN (1994) Nonsurgical management of placenta percreta: a case report. Obstet Gynecol 83(5 pt 2):847–849
Nijman RG, Mantingh A, Aarnoudse JG (2002) Persistent retained placenta percreta: methotrexate treatment and Doppler flow characteristics. BJOG 109(5):587–588
Henrich W, Fuchs I, Ehrenstein T, Kjos S, Schmider A, Dudenhausen JW (2002) Antenatal diagnosis of placenta percreta with planned in situ retention and methotrexate therapy in a woman infected with HIV. Ultrasound Obstet Gynecol 20(1):90–93
Sonin A (2001) Nonoperative treatment of placenta percreta: value of MR imaging. Am J Roentgenol 177(6):1301–1303
Valayatham V, Rao S, Nath R, Raju S, Maxwell D, Oteng-Ntim E (2005) A case of placenta percreta with bladder involvement managed conservatively. J Obstet Gynaecol 25(4):397–398
Arduini M, Epicoco G, Clerici G, Bottaccioli E, Arena S, Affronti G (2010) B-Lynch suture, intrauterine balloon, and endouterine hemostatic suture for the management of postpartum hemorrhage due to placenta previa accreta. Int J Gynaecol Obstet 108(3):191–193
Ferrazzani S, Guariglia L, Triunfo S, Caforio L, Caruso A (2009) Conservative management of placenta previa–accreta by prophylactic uterine arteries ligation and uterine tamponade. Fetal Diagn Ther 25(4):400–403
Hung JH (2007) Pregnancy complicated with maternal pulmonary hypertension and placenta accreta. J Chin Med Assoc 70(6):257–259
Mechery J, Burch D (2006) Alternative management of placenta accreta. Gynecol Surg 3(1):41–42
Shahin AY, Farghaly TA, Mohamed SA, Shokry M, Abd-El-Aal DE, Youssef MA (2010) Bilateral uterine artery ligation plus B-Lynch procedure for atonic postpartum hemorrhage with placenta accreta. Int J Gynaecol Obstet 108(3):187–190
Verspyck E, Resch B, Sergent F, Marpeau L (2005) Surgical uterine devascularization for placenta accreta: immediate and long-term follow-up. Acta Obstet Gynecol Scand 84(5):444–447
Read JA, Cotton DB, Miller FC (1980) Placenta accreta: changing clinical aspects and outcome. Obstet Gynecol 56(1):31–34
Wang LM, Wang PH, Chen CL, Au HK, Yen YK, Liu WM (2009) Uterine preservation in a woman with spontaneous uterine rupture secondary to placenta percreta on the posterior wall: a case report. J Obstet Gynaecol Res 35(2):379–384
Caliskan E, Vural B, Turkoz E, Tan O (2005) Conservative surgical management of placenta percreta: two cases with an emphasis on tubal patency. Gynecol Surg 2(1):29–31
Nagy PS (2003) Spontaneous rupture of the uterus caused by placenta percreta at 28 weeks of twin pregnancy. Eur J Obstet Gynecol Reprod Biol 111(2):207–209
Gupta A, Nanda S, Dahiya P, Chauhan M, Sangwan K (2003) Placenta percreta causing spontaneous uterine rupture in late pregnancy: conservative surgical management. Aust N Z J Obstet Gynaecol 43(4):334–335
Allahdin S, Voigt S, Htwe TT (2011) Management of placenta praevia and accreta. J Obstet Gynaecol 31(1):1–6
Eller AG, Bennett MA, Sharshiner M, Masheter C, Soisson AP, Dodson M, Silver RM (2011) Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol 117(2 pt 1):331–337
Gupta JK, Sinha AS, Lumsden MA, Hickey M (2006) Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 25(1):CD005073
Milam MR, Schultenover SJ, Crispens M, Parker L (2004) Retroperitoneal fibrosis secondary to actinomycosis with no intrauterine device. Obstet Gynecol 104(5):1134–1136
Lim AY, Gaffney K, Scott DG (2005) Methotrexate-induced pancytopenia: serious and under-reported? Our experience of 25 cases in 5 years. Rheumatology (Oxford) 44(8):1051–1055
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11(6):694–703
Shah M, Wright JD (2009) Surgical intervention in the management of postpartum hemorrhage. Semin Perinatol 22(2):109–115
Palacios Jaraquemada JM, Pesaresi M, Nassif JC, Hermosid S (2004) Anterior placenta percreta: surgical approach, hemostasis and uterine repair. Acta Obstet Gynecol Scand 83(8):738–744
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Search Medline
((((((“placenta”[Title/Abstract]) OR “placentas”[Title/Abstract]) OR “placenta’s”[Title/Abstract])) AND ((((((((“increta”[Title/Abstract] OR “increta/percreta”[Title/Abstract])) OR “accreta”[Title/Abstract]) OR “accreta/increta/percreta”[Title/Abstract]) OR “accreta/increta”[Title/Abstract]) OR “accreta/percreta/increta”[Title/Abstract])) OR (“percreta”[Title/Abstract] OR “percreta/increta”[Title/Abstract] OR “percreta involving”[Title/Abstract] OR “percreta placenta”[Title/Abstract] OR “percreta presenting”[Title/Abstract] OR “percreta, placenta”[Title/Abstract])))) AND (((((((((“expectative”[Title/Abstract] OR “expectative approach”[Title/Abstract] OR “expectative attitude”[Title/Abstract] OR “expectative management”[Title/Abstract] OR “expectative policy”[Title/Abstract] OR “expectative treatment”[Title/Abstract])) OR “expectatively”[Title/Abstract]) OR “expectatory”[Title/Abstract])) OR (((“conservative”[Title/Abstract]) OR conventional[Title/Abstract]) OR traditional[Title/Abstract])) OR ((“hysterectomy”[Title/Abstract]) OR “hysterectomies”[Title/Abstract])) OR (((((((((((((“surgery”[Title/Abstract]) OR “surgeries”[Title/Abstract]) OR “resection”[Title/Abstract]) OR “resections”[Title/Abstract]) OR “resections”[Title/Abstract]) OR “surgical”[Title/Abstract]) OR “surgically”[Title/Abstract]) OR “procedure”[Title/Abstract]) OR “procedures”[Title/Abstract]) OR “operation”[Title/Abstract]) OR “operations”[Title/Abstract]) OR “manipulation”[Title/Abstract]) OR “manipulations”[Title/Abstract])) OR (((((((((((((((((“treatment”[Title/Abstract]) OR “treatments”[Title/Abstract]) OR “care”[Title/Abstract]) OR “procedure”[Title/Abstract]) OR “procedure”[Title/Abstract]) OR “procedures”[Title/Abstract]) OR “strategy”[Title/Abstract]) OR “strategies”[Title/Abstract]) OR “usage”[Title/Abstract]) OR “way”[Title/Abstract]) OR “management”[Title/Abstract]) OR “managements”[Title/Abstract]) OR “guidance”[Title/Abstract]) OR “guidances”[Title/Abstract]) OR “support”[Title/Abstract]) OR “therapy”[Title/Abstract]) OR “therapies”[Title/Abstract])).
Search Embase
(placenta:ab,ti OR placentas:ab,ti OR placentas:ab,ti) AND (increta:ab,ti OR accreta:ab,ti OR percreta:ab,ti) AND (expectative:ab,ti OR approach:ab,ti OR attitude:ab,ti OR management:ab,ti OR policy:ab,ti OR treatment:ab,ti OR expectatively:ab,ti OR expectatory:ab,ti OR conservative:ab,ti OR conventional:ab,ti OR traditional:ab,ti OR hysterectomy:ab,ti OR hysterectomies:ab,ti OR surgery:ab,ti OR surgeries:ab,ti OR resection:ab,ti OR resections:ab,ti OR resections:ab,ti OR surgical:ab,ti OR surgically:ab,ti OR procedure:ab,ti OR procedures:ab,ti OR operation:ab,ti OR operations:ab,ti OR manipulation:ab,ti OR manipulations:ab,ti OR treatment:ab,ti OR treatments:ab,ti OR care:ab,ti OR procedure:ab,ti OR procedure:ab,ti OR procedures:ab,ti OR strategy:ab,ti OR strategies:ab,ti OR usage:ab,ti OR way:ab,ti OR management:ab,ti OR managements:ab,ti OR guidance:ab,ti OR guidances:ab,ti OR support:ab,ti OR therapy:ab,ti OR therapies:ab,ti).
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Steins Bisschop, C.N., Schaap, T.P., Vogelvang, T.E. et al. Invasive placentation and uterus preserving treatment modalities: a systematic review. Arch Gynecol Obstet 284, 491–502 (2011). https://doi.org/10.1007/s00404-011-1934-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-011-1934-6