Abstract
Purpose
Human reproduction is a complex process involving multiple factors for the success of pregnancy. Chemokines are one of the immunomodulators which may determine pregnancy outcome. In the present study, we have tested genetic association between CCR5 Δ32 polymorphism and idiopathic recurrent miscarriages (IRM) among north Indians.
Methods
Two hundred patients and 300 age, sex and ethnically matched controls were genotyped for CCR5 Δ32 polymorphism, genotype and allele frequencies were compared in both the groups.
Results
IRM patients had a three times higher (5.5 vs. 1.7%) frequency of heterozygote genotype (P = 0.0335, OR = 3.43; 95% CI = 1.17–10.04). Allele frequency in IRM patients was 3.7 and 0.83% among controls and the differences were statistically significant (P = 0.0349, OR = 3.37; 95% CI = 1.16–9.76).
Conclusions
Our results demonstrated that it had a higher frequency of CCR5 Δ32 at allelic level suggesting a possible susceptibility trend (OR = 3.43) and CCR5 Δ32 may be a potential genetic risk factor for IRM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout history the moral and legal aspects of abortion are subject to intense debate in many parts of the world. About 15–20% of clinically recognized pregnancies end in recurrent miscarriages, the etiology of idiopathic recurrent miscarriages still remains largely unclear till date. Infertility and recurrent miscarriages frequently are causes of worry for women desiring to have children.
Recurrent miscarriage is defined as at least three or more consecutive pregnancy losses before 24 weeks of gestation which affects 0.5–2% of pregnant women [1]. At least 2% of women of reproductive age suffer with two or more consecutive recurrent miscarriages and approximately 1% has three and more pregnancy loss. It occurs often during the first trimester, prior to 13th week of pregnancy.
The causes of recurrent miscarriages is multi-factorial, but can be divided into embryologically driven and maternally driven which affect the endometrium and placental development [2, 3]. Known causes of maternal defects include coagulation disorders, autoimmune defects, endocrine disorders and endometrial defects since there is much debate about cause and association since the exact patho-physiological mechanisms of this disorder are unknown [3]. Therefore, various approaches have been used to study the etiology of miscarriages. Presumed etiological factors include endocrine dysfunction such as hypothyroidism and luteal phase inadequacy, chromosomal aberrations, uterine abnormalities, infectious disorders, and different genetic and environmental factors. These factors are present in, 50% of all women with recurrent miscarriages [4]. There are several reports which emphasize the role of genetic polymorphism and association with IRM.
Chemokines are a large super family of structurally and functionally related cytokines with chemotactic activity targeted at specific leukocyte populations. More than 50 chemokines have been identified till date, but there are large degree of redundancy and overlap of functions [5]. It has been reported that there is a large accumulation of leukocytes in the endometrium in the preimplantation phase of the human menstrual cycle and during early pregnancy [6]. Further, CCR5 is a pro-inflammatory G protein coupled receptors that bind chemokines, such as monocyte chemoattractant protein-1 (MCP-1), regulated on activation, normal T cell expressed and secreted (RANTES) and macrophage inflammatory protein-1 (MIP-1) and has been implicated in atherogenesis [7]. Macrophages comprise 20% of endometrial leukocytes and are present during endometrial proliferation, differentiation and breakdown. There is a marked accumulation of endometrial macrophages specifically in areas of decidualization and trophoblast invasion. These cells are a source of growth factors, cytokines and proteases, creating local microenvironments permissive to tissue remodeling and have been proposed to participate in fetal–maternal interaction at the implantation site [8, 9].
CCR5 is the main cellular receptor for CCL3, CCL4 and CCL5 chemoreceptors. The CCR5 Δ32 is an allelic variant of the CCR5 receptor gene characterized by a 32-bp deletion (CCR5 Δ32) which leads to a non-functional protein and consequently in homozygous individuals there is absence of CCR5 protein expression which may be responsible for the recurrent miscarriages. Therefore, in the present study we investigated the role of CCR5 Δ32 gene polymorphism in women with history of recurrent miscarriages among north Indian women.
Materials and methods
Study design and population
Patients (n = 200) included in the present study were randomly selected from the Department of Medical Genetics, which is one of the super specialty centers in Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS) in Lucknow and from Maharani Laxmi Bai (MLB) Medical College, Jhansi, India. The inclusion criteria for patients included, women with at least three unexplained recurrent miscarriage before 20 weeks of gestation, with the same partner. As per the study design, all these women were investigated to exclude the known causes of recurrent miscarriages (serological tests for toxoplasma, glucose tolerance test, hysterosalpingogram, thyroid function test, luteal phase plasma progesterone assay, anticardiolipin antibody, antiphospholipid antibody, antinuclear antibody test and chromosomal abnormalities of both husband and wife. The chromosomal anomalies of both the partners were done to rule out the male aneuploidies and other chromosomal abnormalities.
The control group consisted of 300 ethnically matched parous women with at least two live births with no history of miscarriage, pre-eclampsia, ectopic pregnancy or preterm delivery (Table 1). All controls were screened for various known causes of miscarriages including, parental chromosomes, day two hormone levels of follicle stimulating hormone (3–11 U/l), leutinizing hormone (3–12 U/l) and testosterone (0.5–3 nmol/l), antiphospholipid antibodies including lupus anticoagulant (PLR 0.8–1.05) and anticardiolipin antibodies (IgG 0–12 GPL units, IgM 0–5 MPL units) and prothrombotic factors like activated protein-C resistance (APCR 2.6–4.36 ratio), factor V Leiden and prothrombin mutations, investigation of leutal phase insufficiency, prolactin dosage, glycaemic curve, thyroid hormone levels, investigation of Toxoplasmosis, Cytomegalovirus, Rubella, HIV, group B Streptococci, Chlamydia trachomatis, hepatitis B and C and bacterial vaginosis. We tried to rule out all the known causes of recurrent miscarriages. A written informed consent was obtained from each participant. Ethical clearance was obtained from SGPGIMS.
Genotyping
For genomic DNA extraction, blood was collected in EDTA. DNA was extracted from blood by using a commercial kit (Qiagen). PCR was used to amplify a segment of the CCR5 gene from 100 ng genomic DNA in a total reaction volume of 25 μl. The primer sequences used for amplification were: forward primer: CCR5-F: TGT TTG CGT CTC TCC CAG and reverse primer: CCR5-R: CAC AGC CCT GTG CCT CTT.
A total of 2.5 mM MgCl2, 200 μM each of dATP, dCTP, dGTP, dTTP, 0.025 units/μl Thermoprime DNA Polymerase, 75 mM Tris–HCl (pH 8.8 at 25°C) 20 mM (NH4)2SO4, 0.16 μM of each forward and reverse primers and 1 μl of template DNA was added per reaction. Polymerase chain reaction was performed using following conditions: 94°C for 5 min, followed by 34 cycles at 94°C for 30 s, 59.3°C for 45 s and 72°C for 1 min followed by final extension at 72°C for 5 min in a thermal cycler. The PCR products were electrophoresed on 2% agarose gel at 120 V for 60 min stained with 0.5 μg/ml ethidium bromide in Tris Borate EDTA (TBE) buffer and visualized by ultraviolet irradiation.
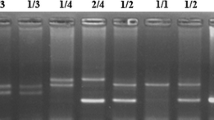
A segment of 193 bp of the CCR5 gene covering the site of deletion was amplified by PCR from the genomic DNA. The amplification produced a single band of 193 bp for the wild type homozygous (+/+) and two bands of 161 and 193 bp because of a 32-bp deletion in the mutant Δ32 allele for the heterozygous (+/Δ) genotype (Fig. 1). Two researchers independently scored the genotypes without the knowledge of disease status of the samples. All the heterozygous samples were re-genotyped using double blind method and the results were noted only for those samples which were reproducible without any discrepancy. Genotyping of 15% of the samples (both in the patient and control groups) were confirmed by DNA sequencing. Upon genotyping 500 samples we observed discrepancy of results in 18 (3.6%) cases. The fresh blood sample was obtained again. The DNA was extracted and the above procedure was repeated. Allele frequencies were determined by direct counting methods.
Statistical analysis
Student’s t test was used to compare means for continuous variables. For each variable, the values are expressed as mean ± SD. The chi-square test was used to assess Hardy–Weinberg equilibrium. The odds ratios with 95% confidence intervals were calculated using standard epidemiological/association methods and significance was assessed by the chi-square test. All P values are two tailed and P values < 0.05 were considered statistically significant.
Results
Characteristics of cases and control in study
The clinical characteristics of the patients and controls analyzed for CCR5 locus are shown in Table 1. The mean age of the controls was 31.9 ± 7.3 years and that of the patients was 28.41 ± 5.9 years. The age range was 20–41 years in patients and 21–42 years in controls.
The genotype distribution in patient and control groups is shown in Table 2. No homozygous individuals for the mutant Δ32 allele were observed in this study. Both groups were in Hardy–Weinberg equilibrium. The frequency of the heterozygous genotype in recurrent miscarriages patients (5.5%) was nearly three times higher than observed in controls (1.7%) with a odds ratio (OR = 3.43, CI = 1.17–10.04) this achieve a statistical significance (P = 0.0335).
The highly significant differences observed among control and patient groups at the genotypic level were also visible at the allelic level (Table 2) as ‘Δ’32 allele was found at frequency of 0.8 among controls and among patients it was found to be 3.7 (P = 0.0349, OR = 3.37; 95% CI = 1.16–9.76). This clearly established that patients with Δ32 genotype are at high risk of developing recurrent miscarriages (OR = 3.43).
Discussion
Besides conferring a strong resistance to homozygote individuals against HIV-1 infection, the potential role of Δ32 allele and other chemokines have been studied by several researchers in a number of other disorders like malignant tumors and their progression, multiple sclerosis, type I diabetes, latent autoimmune diabetes in adults. The data on possible involvement of the CCR5 deletion variant in recurrent miscarriages is not available. To the best of our knowledge, this is the first study from India evaluating the role of CCR5 Δ32 allele in idiopathic recurrent miscarriages.
The mutant allele of the chemokine receptor CCR5 gene (CCR5 Δ32), is believed to have originated from a single mutation event in historic times, and rapidly expanded in Caucasian populations, owing to an unknown selective advantage [10]. This allele is young in evolutionary time, yet it has reached relatively high frequency in Europe. These properties indicate that the mutation has been under intense positive selection. HIV-1 has not exerted selection for long enough on the human population to drive the CCR5 Δ32 allele to current frequencies, fueling debate regarding the selective pressure responsible for rise of the allele. The allele exists at appreciable frequencies only in Europe, and within Europe, the frequency is higher in the north. There are various studies showing the absence of CCR5 Δ32 homozygotes or very less frequency of CCR5 Δ32. Not only there exit differences in the homozygous Δ32 mutants but also there are differences in the heterozygous, i.e. CCR5 +/Δ [10–15] (Table 3). Downer et al. [11] have shown that among United States HIV epidemiology registry which included 1,301 study subjects none of them were homozygous for CCR5 Δ32 mutation, 11.8% of white, 3.7% of black African/Americans, 3.3% of Hispanics/Latinas, 6.6% of other ethnicities were heterozygous. They have proposed these regional differences may be due to racial admixture. Further, the prevalence of CCR5 Δ32 allele in Europe is approximately 10%, and it is low or absent in most of the Asian and African populations [16, 17]. Significant population variation has been documented in Europe ranging from 15% in Iceland to 4% in Greece [18] (Table 3).
In our study, we observed absence of homozygous Δ32 allele, the frequency of heterozygotes in control group was 1.7% which was comparable to previous studies on Indian populations (range 0–6%) [19, 20]. Our data indicated that the heterozygous carriers of CCR5 (+/Δ) gene have ~3.5-fold increased risk of recurrent miscarriages compared to control group. This adds further evidence to the concepts of polygenetic etiological background of women with IRM. Further, no individual was found to be homozygous for mutation (Δ allele) of CCR gene which is comparable to many other studied populations (Table 3). Majumdar and Dey [20] observed that CCR5 Δ32 allele was absent in most ethnic populations of India, except some populations of Northern/Western India where it could have been possibly introduced by Caucasian gene flow. However, in these populations also there was complete absence of homozygous CCR5 Δ32 which is comparable to our results.
Recent identification of a number of chemokines and their receptors at the feto-maternal interface suggest role for chemokines in regulating the processes that occur during implantation [21, 22]. Chemokines and receptor expression was also demonstrated in a number of cell lines representing the components of the feto-maternal interface.
There have been a number of reports describing the expression and regulation of individual chemokines in the endometrium, including IL-8, MCPs-1 and -2, macrophage inhibitory protein (MIP), eotaxin and regulated on activation and normally T cell expressed and presumably secreted (RANTES) [7, 21, 22]. The expression studies were supported by immunolocalization of chemokine protein within the tissue, and the varying cellular localization across the cycle determined. Because chemokines are short lived and are locally acting, identification of cellular location also provides invaluable indicators of function. Leukocyte recruitment has many features in common with trophoblast invasion and trafficking, and it is therefore likely that chemokines play an important role in implantation. During the apposition phase, the blastocysts must find a location on the endometrial epithelium to implant. In the subsequent invasion phase, the trophoblast must traverse first the epithelial basement membrane and then the decidua to reach the uterine blood vessels. Evidences are now accumulating to support a biologically relevant role for chemokines in these processes [23, 24].
Both chemokines and receptors have been identified on invasive cytotrophoblast during the first trimester of pregnancy, and these include CGP-2, stromal cell-derived factor (SDF)-1 and MIP [25] and the receptors CCR1 CCR2B, CCR5, CXCR2B and CX3CR1 [26, 27]. Chemokine receptors (CX3CR1, CCR1, 2 and 3) have also been detected on some trophoblast cell lines [28]. In addition, in floating and anchoring villi, nearly every chemokine targeted for study was expressed by predominantly two cell types, fibroblasts and macrophages. Cytotrophoblast progenitors in floating villi expressed a broad repertoire of chemokine receptors suggesting that cytotrophoblasts are poised to respond to chemokine signals at the maternal–fetal interface [23]. From these studies, it is clear that chemokines may be important determinants of successful implantation and placentation by their actions in chemoattracting leukocytes which are critical players at the embryo–maternal interface, by their actions on trophoblast migration and by additional functions such as cell proliferation and modification of adhesion molecule expression.
The data presented in this study provides evidence for a relationship between IRM and a gene whose gene product is known to influence chemokines and their receptors at the feto-maternal interface might compromise the development of embryo/fetus and its ability to resist maternal alloimmune rejection mechanism. Our data indicate that the investigated CCR5 gene polymorphism confers a small but significantly increased risk for developing IRM. In addition, genetic admixture may be a confounding factor for the association studies, thus only women whose parents were of same ethnicity were included in study and control groups. Various other studies investigating women with IRM used age-matched control to compare genotype frequency. This strategy does not rule out future miscarriages among control groups. To avoid this possible bias, all control patients in our series were postmenopausal at the time of blood sampling.
Conclusions
To conclude, the CCR5 Δ 32 is risk factors for recurrent miscarriages among north Indian population. As technological advances have made sequencing and genotyping cheaper, a more exhaustive screening of the CCR2–CCR5 region is required. In a multifactorial disorder like idiopathic recurrent miscarriages where multiple factors contribute to the pathogenesis, it is very difficult to find a single polymorphism as a susceptibility factor and suggests that gene interaction may contribute to a causal propensity for developing recurrent miscarriages. Hence, more elaborative studies should be undertaken in ethnically diverse groups of the populations that may contribute more towards the functional role of these markers in recurrent miscarriages.
References
Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, CanWild RE (1988) Incidence of early loss of pregnancy. N Engl J Med 319:189–194
Aplin J (2000) Maternal influences on placental development. Semin Cell Dev Biol 11:115–125
Li TC, Makris M, Tomsu M, Tuckerman EM, Laird SM (2002) Recurrent miscarriage, aetiology, management and prognosis. Hum Reprod Update 8:463–481
Clifford K, Rai R, Watson H et al (1994) An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod 9:1328–1332
Rabin RL, Alston MA, Sircus JC, Knollmann-Ritschel B, Moratz C, Ngo D, Farber JM (2003) CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol 171:2812–2824
Salamonsen LA, Woolley DE (1999) Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol 44:1–27. doi:10.1016/S0165-0378(99)00002-9
Gonzalez P, Alvarez R, Batalla A et al (2001) Genetic variation at the chemokine receptors CCR5/CCR2 in myocardial infarction. Genes Immun 2:191–195. doi:10.1038/sj.gene.6363760
Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O (2003) Phenotypic characterization of human decidual macrophages. Clin Exp Immunol 131:498–505. doi:10.1046/j.1365-2249.2003.02092.x
Trundley A, Moffett A (2004) Human uterine leukocytes and pregnancy. Tissue Antigens 63:1–12. doi:10.1111/j.1399-0039.2004.00170.x
Hummel S, Schmidt D, Kremeyer B, Herrmann B, Oppermann M (2005) Detection of the CCR5-32 HIV resistance gene in Bronze Age skeletons. Genes Immun 6:371–374. doi:10.1038/sj.gene.6364172
Downer MV, Hodge T, Smith DK, Qaril SH, Schuman P, Mayer KH, Klein RS, Vlahov D, Gardner LI, McNichol JM (2002) Regional variation in CCR5-Δ32 gene distribution among women from the US HIV Epidemiology Research Study (HERS). Genes Immun 3:295–298. doi:10.1038/sj.gene.6363884
Muxel SM, Borelli SD, Amarante MK, Voltarelli JC, Aoki MN, Coral de Oliveira CE, Maria AEW (2008) Association study of CCR5 delta 32 polymorphism among the HLA-DRB1 Caucasian population in Northern Paraná. Brazil J Clin Lab Anal 22:229–233. doi:10.1002/jcla.20225
Buhler M, Proos A, Howell V, Bennetts B, Burnett L, Stewart G (1998) Evidence from the Australian Ashkenazi Jewish population suggests an eastern European Ashkenazi origin of CCR5-A32 delta 32. Int Conf AIDS 12:148
Yudin NS, Vinogradov SV, Potapova TA, Naykova TM, Sitnikova VV, Kulikov IV, Khasnulin VI, Konchuk C, Vloschinskii PE, Ivanov SV, Kobzev VF, Romaschenko AG, Voevoda MI (1998) Distribution of CCR5-delta 32 gene deletion across the Russian part of Eurasia Hum Genet 102: 695–698
Sharda S, Gilmour A, Harris V, Singh VP, Sinha N, Tewari S, Ramesh V, Agrawal S, Mastana S (2008) Chemokine receptor 5 (CCR5) deletion polymorphism in North Indian patients with coronary artery disease. Int J Cardiol 124:254–258. doi:10.1016/j.ijcard.2006.12.021
Samson M, Libert F, Doranz BJ et al (1996) Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722–725. doi:10.1038/382722a0
Lucotte G (2002) Frequencies of 32 base-pair deletion of the Δ32 allele of the CCR5 HIV-1 co receptor gene in Caucasians: a comparative analysis. Infect Genet 1:201–205. doi:10.1016/S1567-1348(02)00027-8
Petrkova J, Cermakova Z, Lukl J, Petrek M (2005) CC chemokine receptor 5 (CCR5) deletion polymorphism does not protect Czech males against early myocardial infarction. J Intern Med 257:564–566. doi:10.1111/j.1365-2796.2005.01491.x
Ramana GV, Vasanthi A, Khaja M et al (2001) Distribution of the HIV-1 resistance- confring allele SDF-1-3′A, CCR2-64I and CCR5-Delta32 in diverse population Andhra Pradesh, South India. J Genet 80:137–140. doi:10.1007/BF02717909
Majumder PP, Dey B (2001) Absence of the HIV-1 protective Delta CCR5 allele in most ethnic population of India. Eur J Hum Genet 9:794–796. doi:10.1038/sj.ejhg.5200705
Hampton AL, Rogers PA, Affandi B, Salamonsen LA (2001) Expression of the chemokines, monocyte chemotactic protein (MCP)-1 and MCP-2 in endometrium of normal women and Norplant users, does not support a central role in macrophage infiltration into endometrium. J Reprod Immunol 49:115–132. doi:10.1016/S0165-0378(00)00082-6
Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA (2004) Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab 89:6155–6167. doi:10.1210/jc.2004-0507
Drake PM, Red-Horse K, Fisher SJ (2004) Reciprocal chemokine receptor and ligand expression in the human placenta: implications for cytotrophoblast differentiation. Dev Dyn 229:877–885. doi:10.1002/dvdy.10477
Hannan NJ, Jones RL, Critchley HO, Kovacs GJ, Rogers PA, Affandi B, Salamonsen LA (2004) Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestinonly contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J Clin Endocrinol Metab 89:6119–6129. doi:10.1210/jc.2003-031379
Red-Horse K, Drake PM, Gunn MD, Fisher SJ (2001) Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol 159:2199–2213
Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C (2003) Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod 9:189–198. doi:10.1093/molehr/gag024
Sato Y, Higuchi T, Yoshioka S, Tatsumi K, Fujiwara H, Fujii S (2003) Trophoblasts acquire a chemokine receptor, CCR1, as they differentiate towards invasive phenotype. Development 130:5519–5532. doi:10.1242/dev.00729
Hannan NJ, Jones RL, Salamonsen L (2004) Expression of chemokines and their receptors at the human maternal–embryonic interface. Reprod Fertil Dev 16:78
Acknowledgments
We are indebted to the Department of Science and technology, New Delhi for the financial assistance.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parveen, F., Tripathi, G., Singh, B. et al. Association of chemokines receptor (CCR5 Δ32) in idiopathic recurrent miscarriages among north Indians. Arch Gynecol Obstet 280, 229–234 (2009). https://doi.org/10.1007/s00404-008-0901-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-008-0901-3