Abstract
The Investigator Global Assessment of Chronic Hand Eczema (IGA–CHE) is a novel Clinician-Reported Outcome measure that allows investigators to assess cross-sectional CHE global disease severity using clinical characteristics of erythema, scaling, lichenification/hyperkeratosis, vesiculation, oedema, and fissures as guidelines for overall severity assessment. This study aimed to evaluate the psychometric properties of the IGA–CHE for use as an outcome measure in CHE clinical trials and clinical practice. Psychometric analyses were performed using data from a sample of 280 patients with moderate to severe CHE from a phase 3 trial of delgocitinib cream, pooled across treatment groups. Test–retest reliability results were moderate to strong with kappa coefficients ranging from 0.63 to 0.76. Correlations with measures assessing related concepts were moderate or strong (range 0.65–0.72) and exceeded a priori hypotheses, providing evidence of convergent validity. Known-groups validity was supported by statistically significant differences between severity groups (< 0.001). Within-group effect sizes were consistently larger for improved groups compared to stable groups, providing evidence of ability to detect change. Anchor-based analyses generated within-subject meaningful change estimates ranging from – 0.8 to – 2.3. A correlation weighted average suggested a single value of – 1.7 in change from baseline. These findings provide evidence the IGA–CHE scale has strong reliability, construct validity, and ability to detect change, supporting its use as an endpoint in CHE clinical trials and clinical practice. Based on the evidence, 2-level changes in IGA–CHE score are considered a conservative meaningful change threshold; however, findings also indicate 1-level change in IGA–CHE scores reflects a clinically meaningful improvement for patients.
Clinical trial registration: NCT04871711.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic Hand Eczema (CHE) is one of the most frequent chronic inflammatory diseases affecting the hands [1], often caused by contact dermatitis and characterized by poor prognosis [2]. CHE refers to hand eczema that persists for more than 3 months or that returned at least twice within the last 12 months [3]. Currently, there are no topical treatments specifically developed and approved for use in CHE [3]. For trials supporting new drug registrations in dermatological conditions (e.g., atopic dermatitis), regulatory authorities have recommended that an Investigator Global Assessment (IGA) is included as a primary endpoint [4, 5]. An IGA of CHE severity that is valid, reliable, and sensitive to changes over time is, therefore, required to support evaluation of the efficacy of potential treatments for CHE. Such a measure may also be of value as a quick, easily administered method of assessing patient outcomes in clinical practice.
The Investigator Global Assessment of Chronic Hand Eczema (IGA–CHE) is a Clinician-Reported Outcome (ClinRO) measure that allows investigators to assess global disease severity at one given timepoint using clinical characteristics of erythema, scaling, lichenification/hyperkeratosis, vesiculation, oedema, and fissures to guide the overall severity assessment [6]. The IGA–CHE was originally developed by clinical experts, in line with regulatory guidance [7,8,9,10,11], and included in a phase 2b trial (NCT03683719) assessing the efficacy and safety of delgocitinib cream in adult patients with mild to severe CHE [12]. Subsequently, based on regulatory feedback, modifications were made to the IGA–CHE to ensure clear distinction between the severity levels in the descriptions provided. This included updating the definition of ‘almost clear’ from ‘faint erythema’ and ‘no signs of scaling, hyperkeratosis/lichenification, vesiculation, oedema or fissures’ to the presence of ‘barely perceptible erythema’ and ‘no signs of scaling, hyperkeratosis/lichenification, vesiculation, oedema or fissures’. Following these adjustments, evaluation of the psychometric properties of the modified IGA–CHE was considered important to support its use as a clinical trial endpoint and for evaluating CHE severity in clinical practice.
The aim of this research was to evaluate the measurement properties of the IGA–CHE, a new clinician-reported assessment of the severity of CHE signs. This research also aimed to support interpretation of the IGA–CHE scores when used as an outcome assessment in clinical practice or to derive clinical trial endpoints, and ultimately support label claims, awarded by regulators.
Methods
Study design
Data from a phase 3, randomized, double-blind, vehicle-controlled, parallel-group, multi-site trial evaluating the efficacy and confirming the safety of delgocitinib cream in adult patients with moderate to severe CHE (ClinicalTrials.gov ID: NCT04871711) were used for these psychometric evaluation analyses. Patients were assigned to receive delogcitinib cream 20 mg/g or cream vehicle at a ratio of 2:1. Treatment was applied twice daily for 16 weeks. CHE severity was assessed at the trial site by a clinician at screening and then at Weeks 0, 1, 2, 4, 8, 12, and 16 using the IGA–CHE instrument. The primary endpoint was the proportion of patients achieving IGA–CHE treatment success, which was defined as clear (a score of ‘0’) or almost clear (‘1’) from Baseline to Week 16. An institutional review board at each study site approved the study protocol and all activities were conducted in compliance with the International Committee on Harmonization and applicable Good Clinical Practice standards and in accordance with the Declaration of Helsinki and its later amendments.
Participant sample
Subjects were recruited from clinical sites in Canada, France, Germany, Italy, Poland, and the United Kingdom. To be eligible to participate, subjects were required to have a diagnosis of CHE, defined as HE that has persisted for more than 3 months or returned twice or more within the last 12 months, as well as moderate to severe CHE at screening and Baseline according to the IGA–CHE (score of 3 or 4) and a Hand Eczema Symptom Diary (HESD) Itch score [13] (weekly average) of ≥ 4 points for the 7 days preceding Baseline (Full eligibility criteria is provided in Supplemental Table 1). All participants provided written informed consent prior to the conduct of any study activities.
Overview of measures
Investigator Global Assessment of Chronic Hand Eczema (IGA–CHE)
The IGA–CHE is a single item ClinRO that allows investigators to assess overall disease severity at one given timepoint and consists of a five-level severity scale (i.e., 0 = ‘clear’, 1 = ‘almost clear’, 2 = ‘mild’, 3 = ‘moderate’, 4 = ‘severe’) [6]. Each severity level on the scale is clinically characterized in terms of erythema, scaling, hyperkeratosis, vesiculation, oedema, and fissures (Table 1). Assessment is based on the condition of the subject’s disease at the time of evaluation and not in relation to the condition at a previous visit. New lesions on previously untreated areas were included in the assessment. The IGA–CHE for a specific visit is the raw score determined by the clinician.
Convergent validity measures
Other clinician- and patient-reported outcome measures administered alongside the IGA–CHE were used to: a) support evaluation of convergent validity of the IGA–CHE; b) define patients with stable CHE for test–retest reliability analysis; and c) define subjects who experienced change, described in detail below.
Patient Global Assessment of Disease Severity (PaGA). The PaGA is a patient-reported outcome (PRO) global assessment of disease severity in which patients rate their CHE severity on a five-level scale (0 = ‘clear’ [no hand eczema symptoms], 1 = ‘almost clear’ [only slight redness, no other hand eczema symptoms], 2 = ‘mild’, 3 = ‘moderate’, and 4 = ‘severe’) and is based on the severity of a patient’s HE at the time of assessment. The PaGA was completed on an electronic device at the trial site at Baseline and at Weeks 1, 2, 4, 6, 12, and 16.
Hand Eczema Severity Index (HECSI). The HECSI is a ClinRO that clinicians use to rate the severity of six clinical signs of HE (erythema, infiltration/papulation, vesicles, fissures, scaling, and oedema) at the time of evaluation [14, 15]. The HECSI score is calculated by dividing the patient’s hand into five areas (fingertips, fingers, palms, back of hands, and wrists) and the intensity of each of the six clinical signs are measured, using a 4-point severity scale (0 = ‘none/absent’, 1 = ‘mild’, 2 = ‘moderate’, and 3 = ‘severe’). For each location, the area score (total of both hands) is calculated by assigning a score of 0–4 based on the following criteria: 0 = ‘0%’, 1 = ‘1–25%’, 2 = ‘26–50%’, 3 = ‘51–75%’, 4 = ‘76–100%.’ The score given for each location is multiplied by the total sum of the intensity of each clinical feature. Total score ranges from 0 to 360 with higher scores indicating greater severity of CHE. The HECSI was administered at Baseline and Weeks 1, 2, 4, 8, 12, and 16.
Hand Eczema Symptom Diary Patient Global Impression of Severity (HESD PGI-S). The HESD PGI-S is a single item Patient-Global Impression of Severity designed to assess patients’ global perception of the severity of CHE signs and symptoms over the past week and using a 4-point categorical response scale (‘none’, ‘mild’, ‘moderate’, and ‘severe’). The HESD PGI-S was completed on an electronic device at the trial site at Baseline and Weeks 2, 4, 8, and 16.
Hand Eczema Symptom Diary Patient Global Impression of Change (HESD PGI-C). The HESD PGI-C is a single item Patient-Global Impression of Change designed to assess patient perceptions of the overall change in their CHE signs and symptoms since starting the trial treatment, using a 5-point categorical response scale (‘much better’, ‘a little better’, ‘no change’, a little worse’, and ‘much worse’). The HESD PGI-C was completed on an electronic device at the trial site at Weeks 2, 4, 8, and 16.
Statistical methods
Table 2 details the main statistical methods used in this study, designed to evaluate different aspects of IGA–CHE score performance. Other than the cross-tabulation of the IGA–CHE and PaGA, all statistical analyses were detailed a priori in a psychometric analysis plan, finalized prior to receiving the data. The psychometric analysis population, comprised of the first 280 subjects randomized with an IGA–CHE completion at Baseline and Week 16, was used to for all analyses unless otherwise specified. This consisted of a cut of the blinded phase 3 trial data, pooled across delgocitinib cream and cream vehicle groups. All analyses were performed by independent psychometricians not involved with the trial efficacy analyses. Psychometric evaluation was conducted in accordance with best practice guidance from regulators for assessing measurement properties of Clinical Outcome Assessments (COAs) [7,8,9,10,11].
Results
Sample characteristics
Key demographic and clinical characteristics are provided in Table 3. The sample included more female (65.7%) than male subjects and most were white/Caucasian (88.2%) and clinically classified as Fitzpatrick skin types II or III (43.2% and 41.1%, respectively).
Test–retest reliability
The IGA–CHE demonstrated ‘good’ test–retest reliability (kappa coefficients = 0.63–0.69) when subjects were defined as stable based on the PaGA, HESD PGI-S, and HECSI between Weeks 2 and 4. Test–retest reliability was ‘excellent’ (kappa coefficient = 0.76 for all analyses) when subjects were defined as stable on the same measures between Weeks 4 and 8 (Table 4).
Convergent validity
Correlations were examined between the IGA–CHE scores and the PaGA and HESD PGI-S at Week 4 (Table 5). All correlations were moderate or strong (range: 0.65–0.72) and exceeded the hypothesized minimum threshold, providing strong evidence of convergent validity.
Known-groups validity
IGA–CHE scores were compared among groups who differed in their CHE severity as reported on the PaGA and HESD PGI-S (Table 6). There was a pattern of significantly higher mean IGA–CHE scores (indicating worse CHE severity) for subjects who also scored higher (worse) on the PaGA and HESD PGI-S (p < 0.001), with the expected monotonic increases across severity groups. Effect sizes between adjacent groups were moderate to large (ES > 0.71), except for the comparison between the HESD PGI-S “mild” group and “none” group, which had a small effect size (ES = 0.44), but only just below the threshold for moderate. These results provide strong evidence regarding the ability of the IGA–CHE to distinguish patients of clear/almost clear, mild, moderate, and severe severity levels, supporting the construct validity of the IGA–CHE score.
Cross-tabulated tables of categorical IGA–CHE and PaGA scores at Week 8 and Week 16 provide further evidence of known-groups validity. Tables 7 and 8 show these cross-tabulations collapsed in line with the endpoint categories in the phase 3 trial. These results show higher frequencies, where the response options are the same for IGA–CHE and PaGA at Week 8, indicating subjects who scored more severely on the PaGA also scored more severely on the IGA–CHE and vice versa. At Week 16, this is seen for the severe/moderate/mild aligned responses, but a higher frequency of subjects was observed in the PaGA almost clear/clear group with IGA–CHE severe/moderate/mild (n = 40), compared to the almost clear/clear aligned groups (n = 30). However, a low frequency was observed for the PaGA severe/moderate/mild with IGA–CHE almost clear/clear group (n = 7). This indicates subjects scored more severely on the IGA–CHE than the PaGA later in the treatment period. This suggests that the clinicians were rating the patients slightly more severely than patients were rating themselves, perhaps because in the IGA–CHE clinicians base their rating on observable signs, whereas patients will consider pain and itch, which are not observable.
Ability to detect change
Changes in IGA–CHE scores were compared among subjects defined as “improved”, “stable”, and “worsened” on the PaGA, HESD PGI-S, HESD PGI-C, and HECSI between Baseline and Week 16. These results provide evidence that the IGA–CHE can detect change over time, regardless of the rating used to define change. As shown in Table 9, the IGA–CHE score was able to detect improvement, with large effect sizes (ES ≥ 2.79) for the improved group for all anchors. In all cases, the effect size for the stable group was smaller than the improved group, with moderate to large within-group effect sizes (ES range – 0.60 to – 1.10). Differences between change groups were statistically significant (p < 0.001), and between-group effect sizes were large (ES ≥ 1.00) between those defined as improved and stable subjects. Results for the subjects categorized as worsening only showed small changes; however, the sample sizes for the PaGA, HESD PGI-S, and HECSI worsened groups were small (n ≤ 15). The results provide strong evidence for the ability of the IGA–CHE to detect improvement.
Interpretation of scores
Correlations between changes in the IGA–CHE and conceptually similar measures (i.e., PaGA, HESD PGI-S, HESD PGI-C, HECSI-75, and HECSI-90) were all moderate or strong (> 0.50), indicating they are adequately related to support meaningful change analyses. To inform the most appropriate responder definition for the IGA–CHE, the moderately improved group was defined a priori as the group of primary interest for all anchors, except the HESD PGI-C, where the minimally improved group was of primary interest (there was no change level equivalent to moderate improvement due to the HESD PGI-C response scale). As the HECSI-75 and HECSI-90 only have one improvement group counted as responders, this was used as the primary interest group for these anchors.
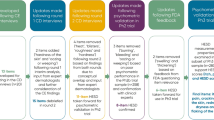
Subjects in the psychometric analysis population who had a minimal improvement on the anchors had mean IGA–CHE score changes between – 0.8 and – 1.0, and subjects who had a moderate improvement on the anchors had mean IGA–CHE score changes between – 1.5 and – 1.6. For the anchor groups of primary interest, subjects had IGA–CHE improvements between – 0.8 and – 2.3 (see Fig. 1). A correlation weighted average with Fisher’s z transformation (accounting for the strength of each anchor’s correlation with the target score) suggested a single value of – 1.7. However, only 2-level or 1-level change is possible for an individual on the IGA–CHE due to the categorical response scale. Empirical Cumulative Distribution (eCDF) and Probably Density Function (PDF) curves supported further examination of both 1-level change and 2-level change as possible responder definitions.
The eCDF and PDF curves showed separation of patients considered minimally improved and moderately improved on the anchors from those considered stable with both a 1-level and 2-level change (see Fig. 2 as an example; the remaining eCDF curves are available in the online supplementary material). Thus, results provide support for both 1-level and 2-level improvement on the IGA–CHE as being appropriate thresholds for defining within-patient clinically meaningful change (noting that a whole level change is required for within-subject change thresholds considering the ordinal nature of the scale). It is suggested that when there is a preference for taking a relatively conservative approach to be very confident of meaningful treatment benefit, a 2-level change on the IGA–CHE can be used as the threshold. As Fig. 2 shows, a 2-level IGA–CHE change would classify < 10% stable subjects as improved according to the HESD PGI-S but also < 50% of moderately improved subjects as improved suggesting it is a high threshold. However, findings also provide support for a 1-level change being important and meaningful to patients. As Fig. 2 shows, a 1-level IGA–CHE change would classify approximately 90% of moderately improved subjects as improved according to the HESD PGI-S, but also approximately 40% of subjects who are stable as improved. Importantly, a 1-level change was well above the distribution-based estimates, indicating that this level of change is above measurement error.
Cross-tabulated ordinal change summaries of IGA–CHE and HESD PGI-S scores support these findings. Table 10 shows that 46.9% of subjects who achieved a 2-level improvement on the HESD PGI-S achieved only a 1-level improvement on the IGA–CHE; 46.9% also a achieved a ≥ 2-level change on the IGA–CHE (summing those with a 2-level improvement on the HESD PGI-S and 2-level, 3-level or 4-level improvement on IGA–CHE). These data provide further evidence that a 2-level change threshold on IGA–CHE is a relatively conservative responder threshold, as less subjects were able to achieve improvement according to this definition compared to the anchor.
Discussion
The aim of this study was to psychometrically evaluate the IGA–CHE to support its use as a clinical trial endpoint as well as in clinical practice for assessing CHE severity. Analyses were performed in accordance with best practices for assessing measurement properties of COAs [7,8,9,10,11]. Findings provide strong evidence supporting the psychometric validity of the IGA–CHE as a comprehensive single-item measure of CHE severity that is reliable and valid, quick and easy to administer, discriminates between patients of differing CHE severity levels and is sensitive to changes in severity over time.
Test–retest reliability results met the threshold for moderate or excellent agreement across the timepoints assessed and regardless of how stability was defined. Although the timepoints used (i.e., 2 and 4 weeks, respectively) are arguably relatively long to expect CHE signs to remain stable, the strength of these results suggest that if it were feasible to examine test–retest over a shorter timeframe the results would be at least equally strong.
Strong or moderate correlations with other measures of related concepts (i.e., HECSI, PaGA and HESD PGI-S), provide evidence of convergent validity and that the IGA–CHE is truly measuring CHE sign severity. Similarly, known groups comparisons showed that the IGA–CHE can distinguish groups of patients who differ in CHE severity on other measures, with statistically significant differences among those groups. The IGA–CHE was also shown to be sensitive to improvements in CHE severity, with large effect sizes within groups defined as ‘improved’ and large between-group differences between ‘improved’ and ‘stable’ groups.
Evidence generated from the anchor-based analyses supports a 2-level change in IGA–CHE scores as a conservative threshold for defining within-subject clinically meaningful change (derived from the – 1.7 single value). Nonetheless, because the analyses suggest a meaningful change threshold could lie anywhere between – 0.8 and – 2.3, the findings also provide support that 1-level change can also be considered an appropriate meaningful change threshold. This was further supported by the distribution-based analyses.
Another example of a single item measure of a patient’s overall CHE severity is the Physician Global Assessment (PGA) used in the alitretinoin studies [26]. Although both IGA–CHE and PGA include five levels, representing 0 = 'clear', 1 = 'almost clear', 2 = 'mild', 3 = 'moderate', 4 = 'severe disease', they differ in that the IGA–CHE uses detailed descriptions within a single scale to characterize each level, whereas for the PGA these levels are defined based on assessment of the severity of each sign or symptom using a separate outcome measure and photo guide. The descriptions for the IGA–CHE were defined carefully, with input from clinical experts and taking account of regulatory feedback, to ensure adjacent levels, in particular ‘almost clear’ and ‘mild’, are clearly distinct. For example, ‘almost clear’ in the IGA–CHE is defined as ‘barely perceptible erythema’ only, whereas definitions of ‘almost clear’ in PGA are broader which may make it more difficult to differentiate between adjacent levels, and potentially lead to inconsistent interpretation. As described above, the known groups findings reported here provide strong evidence this has resulted in a measure that discriminates well between clear/almost clear, mild, moderate, and severe severity levels.
We recognize some limitations in our study. Due to the sample being mainly Canadian and northern European, subjects were predominantly white/Caucasian. Future confirmation of psychometric validity in more racially and ethnically diverse populations would be of value. Furthermore, all psychometric evaluation to-date has been performed in a clinical trial sample. If the IGA–CHE is to be used in real-world studies or in general clinical practice, further evaluation in a ‘real-world’ sample would be beneficial to confirm the measurement properties are fully generalizable in all circumstances.
Conclusion
The IGA–CHE is fit-for-purpose as a valid, reliable, and responsive measure of CHE severity that can be used to support clinical trial endpoints. The IGA–CHE also has value for use in clinical settings to assess CHE severity and monitor clinically meaningful changes in CHE severity over time or in response to treatment.
Data availability
The data sets are available from the corresponding author on reasonable request.
References
Capucci S, Hahn-Pedersen J, Vilsbøll A, Kragh N (2020) Impact of atopic dermatitis and chronic hand eczema on quality of life compared with other chronic diseases. Dermatitis 31(3):178–184. https://doi.org/10.1097/der.0000000000000598
Agner T, Elsner P (2020) Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol 34(Suppl 1):4–12. https://doi.org/10.1111/jdv.16061
Thyssen JP, Schuttelaar MLA, Alfonso JH, Andersen KE, Angelova-Fischer I, Arents BWM et al (2022) Guidelines for diagnosis, prevention, and treatment of hand eczema. Contact Dermatitis 86(5):357–378. https://doi.org/10.1111/cod.14035
FDA/Center for Drug Evaluation and Research (2010) Draft guidance on pimecrolimus. Washington, DC
FDA/Center for Drug Evaluation and Research (2010) Draft guidance on calcipotriene. Washington, DC
Silverberg J, Agner T, Baranowski K, Plohberger U, Thoning H, Arbuckle R et al (2023) Validation of the Investigator Global Assessment of Chronic Hand Eczema (IGA-CHE): a new clinician reported outcome measure [Poster]. EADV Congress
FDA (2009) Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims
FDA (2022) Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments, Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2022. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-selecting-developing-or-modifying-fit-purpose-clinical-outcome
FDA (2023) Patient-Focused Drug Development: Incorporating Clinical Outcome Assessments Into Endpoints For Regulatory Decision-Making: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-incorporating-clinical-outcome-assessments-endpoints-regulatory
FDA (2020) Patient-Focused Drug Development: Collecting Comprehensive and Representative Input: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-collecting-comprehensive-and-representative-input
EMA (2005) Reflection paper on the regulatory guidance for the use of health-quality of life (HRQL) measures in the evaluation of medicinal products. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-health-related-quality-life-hrql-measures-evaluation_en.pdf
Worm M, Thyssen JP, Schliemann S, Bauer A, Shi VY, Ehst B et al (2022) The pan-JAK inhibitor delgocitinib in a cream formulation demonstrates dose response in chronic hand eczema in a 16-week randomized phase IIb trial. Br J Dermatol 187(1):42–51. https://doi.org/10.1111/bjd.21037
Molin S, Seiding Larsen L, Joensson P, Oesterdal ML, Arbuckle R, Grant L et al (2023) Development and psychometric validation of a new patient-reported outcome measure to assess the signs and symptoms of chronic hand eczema (CHE): the Hand Eczema Symptom Diary (HESD). EADV Congress
Held E, Skoet R, Johansen J, Agner T (2005) The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. A study of inter-and intraobserver reliability. Br J Dermatol 152(2):302–307
HECSI [Available from: https://hecsiscore.com/
Fleiss JL, Levin B, Paik MC (2013) Statistical methods for rates and proportions. Wiley
Devji T, Carrasco-Labra A, Qasim A, Phillips M, Johnston BC, Devasenapathy N et al (2020) Evaluating the credibility of anchor based estimates of minimal important differences for patient reported outcomes: instrument development and reliability study. BMJ 369:m1714. https://doi.org/10.1136/bmj.m1714
Hinkle DE, Wiersma W, Jurs SG (2003) Applied statistics for the behavioral sciences. Houghton Mifflin, Boston, Mass, [London]
Hedges LV (1981) Distribution theory for glass’s estimator of effect size and related estimators. J Educ Stat 6(2):107–128. https://doi.org/10.2307/1164588
Cohen J (2013) Statistical power analysis for the behavioral sciences. Academic press
Kazis LE, Anderson JJ, Meenan RF (1989) Effect sizes for interpreting changes in health status. Med Care 27(3 Suppl):S178–S189. https://doi.org/10.1097/00005650-198903001-00015
Revicki D, Hays RD, Cella D, Sloan J (2008) Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 61(2):102–109. https://doi.org/10.1016/j.jclinepi.2007.03.012
Trigg A, Griffiths P (2021) Triangulation of multiple meaningful change thresholds for patient-reported outcome scores. Qual Life Res 30(10):2755–2764. https://doi.org/10.1007/s11136-021-02957-4
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41(5):582–592. https://doi.org/10.1097/01.Mlr.0000062554.74615.4c
Osoba D (2005) The clinical value and meaning of health-related quality-of-life outcomes in oncology. In: Gotay C, Snyder C, Lipscomb J (eds) Outcomes assessment in cancer: measures, methods and applications. Cambridge University Press, Cambridge, pp 386–405
Ruzicka T, Lynde CW, Jemec GB, Diepgen T, Berth-Jones J, Coenraads PJ et al (2008) Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol 158(4):808–817. https://doi.org/10.1111/j.1365-2133.2008.08487.x
Acknowledgements
We thank Alyson Young, PhD at Adelphi Values, Patient-Centered Outcomes for her medical writing support funded by LEO Pharma A/S.
Funding
The study was funded by LEO Pharma.
Author information
Authors and Affiliations
Contributions
All authors contributed to the interpretation of the results reviewed and provided input into multiple drafts of the manuscript and approved the final manuscript. GS performed the analyses. RA, LG and GS contributed to the design and conception of the study, were involved in drafting the analysis plan.
Corresponding author
Ethics declarations
Conflict of interest
JS: has received honoraria as a consultant and/or advisory board member and/or speaker and/or received grants from AbbVie, Alamar, Aldena, Aobiome, Arcutis, Arena, Asana, Aslan, BioMX, Biosion, Bodewell, Boehringer-Ingelheim, Bristell-Myers Sqibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, Corevitas, Dermavant, Dermtech, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma A/S, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sanofi-Genzyme, Shaperon, TARGET-RWE, Union.. TA: has given lectures, participated in clinical studies, or been on advisory boards for Sanofi, LEO Pharma, Pfizer, Eli Lilly, Galderma, and AbbVie. KB, UP, HT: are employees of LEO Pharma A/S, Ballerup, Denmark. RA, LG, GS: are employees of Adelphi Values, Patient-Centered Outcomes, Cheshire UK, and Adelphi Values was contracted by LEO Pharma to support them in performing the psychometric analyses. RB: is an advisory board member, consultant, speaker and/or investigator for and receives honoraria and/or grants from AbbVie, Arcutis, Arena Pharma, Asana BioSciences, Bellus Health, Boehringer-Ingelheim, CARA, Dermavant, Eli Lilly, EMD Serono, Galderma, Incyte, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Pfizer, Ralexar, RAPT, Regeneron, Sanofi Genzyme, and Sienna. He is also an employee and shareholder of Innovaderm Research.
Ethical approval
The trial was conducted in accordance with the consensus of ethical principles from internal guidelines including the Declaration of Helsinki, Council for Internal Organization of Medical Sciences (CIOMS) International Ethical Guidelines, International Council of Harmonization Good Clinical Practice (ICH GCP) Guideline, EU General Data Regulation 2016/679 of 27 April 2016 and in compliance with the approved protocol and applicable laws and regulations. All procedures involving study participants were approved by the institutional review board or independent ethics committees at all investigational sites.
Informed consent
Written informed consent to participate in the trial and publication was obtained from all subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silverberg, J.I., Agner, T., Baranowski, K. et al. Validation of the Investigator Global Assessment of Chronic Hand Eczema (IGA–CHE): a new clinician reported outcome measure of CHE severity. Arch Dermatol Res 316, 110 (2024). https://doi.org/10.1007/s00403-024-02818-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-02818-3