Abstract
Studies have demonstrated that bleach baths improve atopic dermatitis (AD) severity; however, the effects on itch, skin barrier, and cutaneous microbial composition are less clear. We examined whether bleach baths reduce itch, normalize skin barrier function, reduce S. aureus absolute abundance, and increase microbial diversity in adults with AD who were colonized with S. aureus on their non-lesional skin. This was an open label, non-randomized, controlled trial performed at a single academic center. Fifteen AD and five non-atopic healthy controls (NA) were instructed to take two bleach baths (0.005% NaClO; 5–10 min duration) per week for a total of 12 weeks as add-on therapy. Adults 18 to 65 years (inclusive) with mild to severe AD were recruited with EASI score > 6.0, S. aureus culture positivity, access to a bathtub, and ability and willingness to maintain current topical or systemic treatments. They were evaluated at baseline (before bleach baths), 6 weeks, and 12 weeks after the intervention of twice-weekly bleach baths. Efficacy measurements included EASI as well as 5-D Pruritus and ItchyQoL™. Transepidermal water loss (TEWL) and stratum corneum (SC) integrity assay were performed to assess the skin barrier. Skin dysbiosis was measured by S. aureus cultivation, S. aureus abundance (qPCR of thermonuclease gene), and V1-V3 16S rRNA gene sequencing on non-lesional and lesional AD skin. After 12 weeks of bleach baths, 8/15 (53.3%) AD subjects achieved an EASI50 and a significant reduction in itch as measured by 5-D pruritus and Itchy QoL. Eighty-seven percent reported improvements in sleep quality. At study entry, AD subjects had higher non-lesional TEWL values than NA subjects, and only AD subjects experienced a reduction with bleach baths (p = 0.006). Similarly, SC integrity improved as early as 6 weeks after bleach baths in AD subjects. Notably, bleach baths had no significant effect on S. aureus culture-positivity, qPCR absolute abundance, or microbial diversity. The addition of twice-weekly bleach baths improves investigator-assessed AD severity, patient-reported pruritus and sleep as well as physiological measures of skin barrier function in adult AD subjects while having no effect on qualitative and quantitative measures of cutaneous S. aureus. Trial Registration: ClinicalTrials.gov Identifier: NCT01996150, Date of registration: November 27th, 2013.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease, affecting 15–20% of children and 1–3% of adults worldwide [1]. It is characterized by skin barrier disruption, type 2 immunity, and reduced quality-of-life (QoL) resulting from the itch-scratch cycle. AD patients also exhibit skin bacterial dysbiosis with high rates of Staphylococcus aureus (S. aureus) colonization especially in subjects with more severe disease and greater type 2 polarization [2,3,4,5,6]. Whether disease severity begets S. aureus colonization or chronic colonization leads to the worsening of the disease is not clear. Safe and cost-effective topical therapies for the management of AD are still limited and whether therapies that primarily act by normalizing skin dysbiosis are effective has not been adequately tested.
Staphylococcus aureus is not part of the normal human skin flora, but can be cultured from lesional skin in up to 100% of AD subjects and is strongly linked to AD flares and severity [2, 5, 7,8,9]. This S. aureus colonization is thought to be due to decreased levels of antimicrobial peptides and the expression of S. aureus adhesins [10, 11]. Virulence factors released by S. aureus are thought to play a role in skin barrier disruption and their effects are enhanced in the presence of type 2 cytokines [12, 13]. Current guidelines support the use of antistaphylococcal antibiotics to treat overt skin infections but are not recommended to treat the staphylococcal colonization commonly seen in AD subjects [14].
Several topical antistaphylococcal approaches, including antibacterial soaps, antibacterial bath additives, and bleach baths have been claimed to improve AD severity and/or decrease secondary infections, but only bleach baths have become “standard of care” as an add-on therapy [15, 16]. This treatment first gained attention when a 2009 placebo-controlled study found that children with S. aureus-infected, moderate-severe AD exhibited significant reductions in disease severity as measured by the Eczema Area and Severity Index (EASI) after an oral antibiotic course and 3 months of maintenance therapy with twice-weekly bleach baths (0.005% sodium hypochlorite [NaClO]) used in conjunction with monthly intranasal mupirocin [17]. Since then, several studies have also observed that bleach baths improve disease severity [18,19,20,21,22]. The 2009 study found that contact with the bleach-containing water was important, as the best improvements were seen with submerged body parts. However, no change in S. aureus-positivity was observed [17].
The mechanism of action for bleach baths in AD remains unclear. Studies have shown that a much higher concentration of NaClO (0.04%) is needed to reduce S. aureus derived from AD skin ex vivo [23]. One study found no bacteriocidal effect of NaClO on S. aureus (or S. epidermidis) growth (log phase or stationary) until > 0.03% concentration [24]. This group further modeled the effect of 0.005% NAClO on S. aureus CFUs in porcine skin, where they also found no bactericidal effect [24]. Further study is required to better understand the effects of bleach baths on the entire skin microbial community. It is possible that bleach baths may have an anti-inflammatory effect, as NaClO has been shown to decrease the expression of NF-κB associated genes in human keratinocytes [25].
In addition, there is limited data on the effects of bleach baths on barrier function in AD. Shi et al. conducted a split-body, randomized, controlled trial and found that bleach baths do not significantly affect transepidermal water loss (TEWL), stratum corneum (SC) hydration, or pH compared to tap water baths when assessed at baseline and up to 60 min post bleach bath immersion [26]. Further, a randomized, placebo-controlled crossover trial of 40 pediatric subjects with AD found no significant difference in TEWL or SC hydration after 4 weeks of at least twice-weekly bleach bath treatment versus tap water, however, this study had a high non-adherence rate (n = 14) [27]. Little is known about how barrier function is affected in the presence of a longer bleach bath intervention period.
To assess whether bleach baths normalize skin barrier function and dysbiosis and/or reduce itch, we performed an open-label trial in adult AD subjects colonized with S. aureus. These parameters were studied at baseline and after 6 and 12 weeks of 0.005% sodium hypochlorite baths twice-weekly. We compared these observations with what was observed in a cohort of non-atopic (NA) healthy adult controls, treated in parallel with the same bleach bath regimen.
Results
Bleach baths reduce atopic dermatitis disease severity
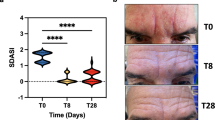
The population demographics and study flow diagram are summarized in Table 1 and Fig. SI available in the electronic supplementary material (ESM). A significant absolute reduction in EASI score was observed by 6 weeks (31.4% improvement) and was slightly greater after 12 weeks (50.5% improvement) of treatment (Fig. 1a). By 6 and 12 weeks, 33% (5/15) and 53% (8/15) of subjects, respectively, reached a reduction of at least 50% in the EASI score (EASI50) (Fig. 1b). The majority (75%) of the severe AD subjects surpassed the minimal clinically important difference (MCID), defined as a reduction of ≥ 6.6 points from baseline EASI [28], after 6 weeks of bleach baths, while a smaller percentage (25%) of subjects with moderate disease at baseline achieved an MCID at 6 weeks (ESM Table SI). Although serum total immunoglobulin E (IgE) and Type 2 chemokine biomarkers PARC/CCL18 and TARC/CCL17 are considered good predictors of AD severity [29, 30], they were not predictive of bleach bath efficacy in our study cohort (ESM Fig. S2).
Bleach bath treatment reduces atopic dermatitis disease severity and itch. (a) The absolute EASI of AD subjects before and after 6 and 12 weeks of bleach bath treatment are shown as well as the (b) percent improvement in EASI. The dotted line represents a 50% improvement, or EASI50. For (a) data are mean ± SD, n = 15 AD subjects, **P = 0.0057, ***P = 0.0002 by One-way ANOVA with Dunn’s multiple comparisons test. For (b) data are mean percent improvement of n = 15 AD subjects at 6 and 12 weeks. Colors represent the same subjects over time. Itch was quantified using the (c, d) 5-D Pruritus and (e) ItchyQoL™ questionnaires at the time of enrollment and after 6 and 12 weeks of bleach bath treatment. (d) The 5-D Pruritus sleep score values are: 1 = never affects sleep, 2 = occasionally delays falling asleep, 3 = frequently delays falling asleep, 4 = delays falling asleep and occasionally wakes me up at night, 5 = delays falling asleep and frequently wakes me up at night. Data are mean ± SD, n = 15 AD subjects. For (c) *P = 0.0105, ***P < 0.0001 (d) **P = 0.002 and (e) ***P < 0.0001 by One-way ANOVA with Dunn’s multiple comparisons test. Colors represent the same subjects over time
Bleach baths reduce itch
AD subjects rated their itch at each visit by answering both the 5-D Pruritus and ItchyQoL™ questionnaires. The mean 5-D pruritus score was significantly reduced after 6 and 12 weeks of bleach baths (Fig. 1c). Sleep was ranked as one of the highest disabilities (5-D Pruritus subscore) in 12/15 subjects (80%) and this measure was significantly improved by 12 weeks in 87% of subjects (13/15) (Fig. 1d). Similarly, the ItchyQoL™ questionnaire revealed a clear trend of itch reduction after 6 weeks with 80% of subjects (12/15) noting decreased itch (P = 0.06) which became significant after 12 weeks of bleach baths, with 100% of subjects noting improvements in itch (Fig. 1e).
Bleach baths improve skin barrier function
Baseline TEWL values were significantly higher in AD subjects’ non-lesional skin compared to NA on study entry (14.5 ± 5.1 vs. 8.9 ± 2.9; P = 0.0298; Fig. 2a), indicating greater barrier dysfunction [31]. Baseline TEWL values significantly decreased in AD subjects but did not change in NA subjects with bleach bath treatment (Fig. 2a). Previous studies have suggested that AD skin has reduced SC hydration and higher pH compared to non-atopic skin [32]. However, we found no significant difference in these measures between AD and NA subjects on study entry and, importantly, no change following bleach baths (ESM Fig. S3a, b).
Bleach bath treatment improves skin barrier function. (a) TEWL in NA and AD subjects at baseline (pre-tape-stripping). (b) TEWL in NA and (c) AD at baseline and after 5, 10, and 15 tape-strips before treatment (black), after 6 weeks (red) and 12 weeks (blue) of bleach baths. (d) Normalized Area under the curve (AUC) calculations for TEWL values. Data are mean ± SD, n = 15 AD subjects and n = 5 NA subjects. For (a) *P = 0.0298 by Two-way ANOVA with Sidak’s multiple comparisons test, **P = 0.0121, ***P = 0.0043 by Two-way ANOVA with Tukey’s multiple comparisons test. For (d) *P = 0.01 by Two-way ANOVA with Sidak’s multiple comparisons test, **P = 0.0059, ***P = 0.0055 by Two-way ANOVA with Tukey’s multiple comparisons test. Colors represent the same subjects over time
Bleach baths significantly improve SC integrity in AD subjects
We evaluated SC integrity in non-lesional skin by measuring TEWL after 5, 10 and 15 sequential tape-strippings and reported the observation as an area under the curve (AUC) [33]. In a compromised skin barrier characteristic of AD, the AUC of the sequential TEWL measurements is greater than what is observed in healthy subjects [34]. In NA subjects, SC integrity was not altered after 6 or 12 weeks of bleach baths (Fig. 2b). AD subjects, however, experienced much more significant increases in TEWL after tape-stripping on study entry (black line, Fig. 2c), indicative of an SC defect [35, 36]. Bleach baths significantly improved SC integrity in AD subjects after 6 and 12 weeks (Fig. 2c). When the data is normalized to account for differences in baseline values (pre-tape-stripping) between NA and AD subjects, a significant difference between NA and AD AUC values at study entry is noted (Fig. 2d). However, only AD subjects had a significantly decreased AUC after 6 and 12 weeks of treatment (Fig. 2d).
S. aureus growth and abundance is not altered by bleach baths
Bleach baths did not reduce S. aureus on the skin, and the majority of AD subjects remained S. aureus-culture positive at non-lesional, lesional, or both skin sites after 12 weeks of bleach baths (data not shown). All subjects in the study were negative for methicillin-resistant S. aureus (MRSA). We performed qPCR and quantified the copy number of the thermonuclease (nuc) gene, which is specific for S. aureus and is a measure of the absolute abundance of both replicating and nonreplicating S. aureus. No significant changes were found in S. aureus abundance on non-lesional or lesional skin in NA or AD subjects following bleach baths (Fig. 3a).
Bleach baths have no significant effect on S. aureus abundance or the skin microbiome. (a) S. aureus abundance (Log10 nuc gene copy number by qPCR) and (b) relative abundance (% operational taxonomic units [OTU] by high-throughput sequencing) were measured from skin swabs of normal skin of NA and non-lesional and lesional skin of AD taken before and after 6 and 12 weeks of bleach baths. (c) Bacterial taxonomic classifications at the species level from NA and AD non-lesional and AD lesional skin swabs before and after taking 6 and 12 weeks of bleach baths. (d) Shannon diversity analyses were performed using QIIME
Bleach baths do not alter skin microbial diversity
We performed high-throughput sequencing of the variable regions, V1-V3, of the conserved 16S rRNA gene to obtain in-depth profiling of the skin microbiome on non-lesional (NA and AD) and lesional (AD) skin before and after bleach baths. The relative abundance of major genera and species is shown in ESM Fig. S4 and Fig. 3c. The mean ± SD of the distribution of the top 10 bacterial taxa at the species level is shown in ESM Table SII. We observed a significantly higher relative abundance [% Operational Taxonomic Units (OTU)] of S. aureus in AD non-lesional and lesional skin compared to NA skin at all time points (Fig. 3b, c). However, the % OTU of S. aureus was not significantly altered on AD non-lesional and lesional skin after taking bleach baths (Fig. 3b, c).
We also assessed whether bleach baths affected the microbial dysbiosis associated with AD skin by evaluating several measures of alpha diversity. The Shannon Diversity Index was unchanged at both non-lesional and lesional sites (Fig. 3d). The significant decreases in absolute EASI scores and itch did not significantly correlate with the Shannon Diversity Index, suggesting that improvements may not be driven by the relative changes in skin microbial populations (data not shown). Bleach baths had no effect on other measures of phylogenetic diversity including Chao1, PD Whole Tree and Strong Dominance Index (ESM Fig. S5a–c). Collectively, these data show that 12 weeks of bleach bath treatments do not significantly alter S. aureus cultivability and absolute abundance, or alter the relative abundance of the major bacterial skin communities. Furthermore, this highlights that reductions in absolute or relative amounts of S. aureus from AD skin may not be necessary to improve AD severity and symptomatology.
Discussion
This study demonstrated that the addition of twice-weekly bleach baths in an adult, S. aureus-positive AD population improves disease severity, reduces itch and and itch-driven QoL measures, and enhances skin barrier functions. Notably, these improvements were seen without any reductions in S. aureus skin colonization measured by highly quantitative methods, suggesting that bleach baths improve AD through another mechanism. Importantly, bleach baths had no effect on skin hydration or pH in AD and NA subjects, suggesting that the alkalinity of bleach baths did not dry out the skin or worsen the abnormally elevated skin pH characteristically observed in AD subjects.
Our observation that bleach baths significantly reduce disease severity is in accordance with many studies that demonstrated significant improvements in clinical disease after bleach bath treatment [17,18,19,20,21,22]. As 75% of our study subjects with severe disease reached the MCID after 6 and 12 weeks, this highlights the possibility that bleach baths may be more effective in subjects with more severe disease or simply reaffirms that it is easier to achieve an MCID with a higher baseline EASI score. Further, Bakaa et al. noted a 22% relative decrease in AD severity with bleach bath treatment, resulting in a more pronounced decrease in EASI score for subjects with high disease activity compared to subjects with lower disease activity at baseline [37]. In contrast, Hon et al. found that four-week, twice-weekly bleach baths were not more useful than taking water baths in improving AD signs and symptoms in children [27]. Reasons for the discrepancies may be greater non-adherence to bleach baths or robustness of patient-reported measures in this younger population, the concomitant administration of oral antibiotics, or simply real biological differences in response to bleach in young versus adult AD skin [27].
The dominant symptom experienced by AD subjects is pruritus [38] which leads to a significant reduction in QoL [39]. Although remarkably little is known about what controls the intense itch associated with AD, several factors have been linked to itch, including prostaglandins, serotonin, beta-endorphins, proteases, and certain cytokines [38, 40]. Fukuyama et al. stimulated the dorsal root ganglia from mice treated with topical hydrochlorous acid or betamethasone dipropioinate with both histaminergic and non-histaminergic stimuli and found significantly less sensory nerve activation compared to untreated mice, suggesting that hypochlorous acid may play a role in sensory nerve desensitization. This study also showed that hypochlorous acid exhibits broad anti-inflammatory effects via decreased phosphorylation of MAP kinases and IkB pathways in dendritic cells after LPS exposure. However, these effects were specific to hypochlorous acid (HOCl), not hypochlorite (OCl-) which is the predominant form in bleach [41]. Notably, it was also found that itch intensity correlated with measures of skin barrier dysfunction (e.g. increased TEWL and decreased SC hydration) [42]. Consistent with this, we observed significant correlations between baseline TEWL and 5-D Pruritus and ItchyQoL™ scores (ESM Fig. S6a, b) in our study. Whether this suggests that the itch/scratch cycle is a key determinant of the skin barrier defect or that a disrupted barrier induces itch has yet to be determined. We also observed a strong correlation between baseline TEWL and EASI (ESM Fig. S6c), suggesting that bleach baths may improve disease severity by reducing itch and enhancing skin barrier function. Wong et al. found a significant reduction in itch by visual analog scale in AD subjects after two months of treatment with bleach baths, however, this improvement was no greater than that of the water bath control group [19].
SC dysfunction in AD is partially due to alterations in lipid composition/conformation [43, 44], reduced expression of structural proteins [45, 46], and simply the mechanical trauma of scratching [38]. Our data demonstrates that bleach baths improve barrier function, as seen by basal TEWL and SC integrity measurements (Fig. 2), which is in contrast to previously reported effects of bleach baths on barrier function [26, 27]. Our results indicate that the skin is less “leaky” (Fig. 2a) and suggest that bleach enhances corneocyte adhesion and/or improves the composition or organization of lipid lamellae. A previous study has shown that exposure to 0.05% diluted hypochlorite did not change ceramide or non-ceramide fractions of skin lipids in canine epidermal cell culture [47], which may suggest that positive effects of bleach baths on skin barrier are independent of skin lipid abnormalities common in AD. Future studies are needed to address the mechanism responsible for skin barrier enhancement and should include assessment of the skin transcriptome and lipidome.
Our results are consistent with other studies that noted improvements in AD with no significant reduction in S. aureus colonization after bleach baths [17, 19, 22]. Two of the studies used the same concentration and regimen as we used, while one used a 0.006% NaClO body wash for 6 weeks of daily use. Despite no differences in S. aureus colonization, Wong et al. observed a reduction in S. aureus density of 41.9% after 1-month and 53.3% after 3-months in children and young adults [19]. Huang et al. observed no significant reduction in S. aureus colonization at 1 month and 3 months despite 10 days of cephalexin therapy for baseline infection and monthly intranasal mupirocin treatment in conjunction with bleach baths [17]. Others have found recolonization within four weeks of stopping aggressive oral and topical antimicrobial treatments [7], suggesting the possibility of recolonization from body reservoirs such as the nares, perineum, or pharynx [48], or from close contact with partners and even household pets [49].
Gonzalez et al. were the first to analyze the skin microbiome of non-lesional and lesional skin in young children with and without AD and compare changes after treatment with topical corticosteroids alone or in conjunction with bleach baths [18]. Both treatments normalized lesional AD skin to more closely resemble non-lesional AD skin by the restoration of microbial diversity, decreased total bacterial burden, and decreased density of Staphylococcus species; however, both were still distinctly different than controls. Since their study did not include a group that took only bleach baths, we cannot know the relative contribution of bleach baths versus corticosteroids to the observed effects.
Our observation that bleach baths did not significantly alter the AD skin microbiome, despite improving severity, was unexpected. Since our AD subjects were required to be S. aureus-positive to be enrolled in our study, it was not surprising that we could detect S. aureus by culture, qPCR, and sequencing. Prior studies have shown selective shifts in the relative abundance of S. aureus during AD flares, with S. aureus reaching up to 90% of the total bacterial population [2]. We observed a more modest shift in the Staphylococcus genus or S. aureus species when comparing AD lesional with non-lesional skin or NA skin (Fig. 3c). We observed a high degree of variability between subjects, as noted by the large standard deviations, consistent with the published literature (ESM Table SII) [50]. Although we found no difference in S. aureus abundance, it is possible that bleach baths changed or reduced S. aureus toxins or virulence factors, which were not measured in our study.
In summary, our study confirms that bleach baths are safe and well-tolerated, corroborates published work demonstrating the ability of bleach baths to improve AD severity and itch, and is the first to show significant improvements in patient-reported outcomes of itch-related QoL, namely sleep, after bleach bath treatment. Importantly, the itch benefit (and EASI) correlated with improvements in skin barrier function and not the quantity of S. aureus on the skin surface (ESM Fig. S6). Future studies are needed to understand the mechanism(s) responsible for this bleach bath-induced skin barrier and itch improvement and to determine whether changes precede improvements in the signs of the disease (EASI).
Material and methods
Study population
Adults 18–65 years (inclusive) with mild-severe AD were recruited. Inclusion criteria included: EASI score greater than 6.0, S. aureus culture positivity, access to a bathtub, and willingness to maintain a stable dose of topical or systemic treatments for the duration of the study. Fifteen AD and five NA subjects completed all visits and met compliance requirements. NA were defined as having no personal or first-degree relative with a history of atopy and were comparable to the AD group in age, gender, and race. All NA subjects were negative for S. aureus by standard culture techniques. The study protocol was approved by the University of Rochester Medical Center RSRB (NCT01996150).
Study design
Study participants were each given a bottle of 8.25% concentrated Clorox® bleach with a measuring cup marked to 3-oz volume. Participants were instructed to dilute 3 oz of bleach in approximately 40 gallons of water (final concentration 0.005% NaClO in a standard bathtub) and bathe for 5–10 min, 2x/week for a total of 12 weeks. Subjects were evaluated at 3 different time points: baseline (before bleach baths), 6 and 12 weeks after bleach baths.
Assessments
EASI
EASI was used to measure the extent and severity of AD. Mild disease is defined by EASI scores of 1.1–7.0, moderate by 7.1–21.0, severe as 21.1–50.0, and very severe 50.1–72 [51]. No subjects enrolled met the very severe category. The MCID for EASI is calculated by the difference between EASI scores.
Itch
Pruritus was quantified using two validated patient-reported questionnaires: 5-D pruritus scale (Scale 5–25) [52] and ItchyQoL™ (Scale 22–110) [53]. The 5-D pruritus scale scores itch in 5 domains: 1. duration (how many hours spent itching), 2. degree (itch intensity), 3. direction (itch improved or worsened compared to the previous month), 4. disability (quality of life), and 5. distribution (body part). ItchyQoL™ is a 22-item questionnaire that assesses the quality of life related to itch in three main domains: symptoms, functioning, and emotions.
Skin barrier measurements
TEWL (Aquaflux, Biox Systems Ltd, London, UK), pH, and skin hydration measurements (Courage & Khazaka, Cologne, Germany) were obtained from a non-lesional site on the non-sun-exposed volar forearm in both AD and NA subjects prior to and after repeated tape-strippings (5, 10 and 15 Cuderm™ tape applications) using the Cuderm™ pressure device [54, 55]. This site could not be treated with medications or moisturizers within the previous 24 h.
S. aureus culture
Skin swabs were collected to determine S. aureus colonization and methicillin resistance using standard culture methods in the CLIA-certified URMC Clinical Microbiology Laboratory. Non-lesional sites were selected immediately adjacent to sites on the volar (non-sun-exposed) forearm where skin barrier functions were performed. Lesional sites were selected from the same arm when possible. A 3 × 3 cm area of skin was swabbed using non-bacteriostatic saline moistened rayon-tipped BBL™ CultureSwabs™ (Becton, Dickinson and Company, Le Pont de Claix, France).
S. aureus abundance and microbiome
Skin swabs were collected as noted above and processed to extract total genomic DNA as previously described [56] using DNA extractions, MasterPure Yeast DNA Purification Kit (Epicentre, Madison, WI) and PureLink Genomic DNA Mini Kit (Invitrogen) (see ESM).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Eichenfield LF (2004) Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy 59(Suppl 78):86–92. https://doi.org/10.1111/j.1398-9995.2004.00569.x
Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA et al (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22(5):850–859. https://doi.org/10.1101/gr.131029.111
Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D et al (2016) Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol 137(4):1272–1274. https://doi.org/10.1016/j.jaci.2015.07.052
Leyden JJ, Marples RR, Kligman AM (1974) Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol 90(5):525–530. https://doi.org/10.1111/j.1365-2133.1974.tb06447.x
Simpson EL, Villarreal M, Jepson B, Rafaels N, David G, Hanifin J et al (2018) Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype. J Invest Dermatol 138(10):2224–2233. https://doi.org/10.1016/j.jid.2018.03.1517
Simpson EL, De Benedetto A, Boguniewicz M, Ong PY, Lussier S, Villarreal M et al (2023) Phenotypic and endotypic determinants of atopic dermatitis severity from the Atopic Dermatitis Research Network (ADRN) Registry. J Allergy Clin Immunol Pract. https://doi.org/10.1016/j.jaip.2023.04.052
Breuer K, HÄussler S, Kapp A, Werfel T (2002) Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol 147(1):55–61. https://doi.org/10.1046/j.1365-2133.2002.04872.x
Warner JA, McGirt LY, Beck LA (2009) Biomarkers of Th2 polarity are predictive of staphylococcal colonization in subjects with atopic dermatitis. Br J Dermatol 160(1):183–185. https://doi.org/10.1111/j.1365-2133.2008.08905.x
Simpson EL, Schlievert PM, Yoshida T, Lussier S, Boguniewicz M, Hata T et al (2023) Rapid reduction in Staphylococcus aureus in atopic dermatitis subjects following dupilumab treatment. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2023.05.026
Cho SH, Strickland I, Boguniewicz M, Leung DY (2001) Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol 108(2):269–274. https://doi.org/10.1067/mai.2001.117455
Donald L, Bissonnette R, Berdyshev E, Kreimer S, Lyub-Chenko T, Bafna S et al (2023) Dupilumab inhibits vascular leakage of blood proteins into atopic dermatitis skin. J Allergy Clin Immunol 151(2):Ab147-Ab
Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL et al (2019) The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol 143(1):26–35
Bin L, Kim BE, Brauweiler A, Goleva E, Streib J, Ji Y et al (2012) Staphylococcus aureus alpha-toxin modulates skin host response to viral infection. J Allergy Clin Immunol 130(3):683-91e2. https://doi.org/10.1016/j.jaci.2012.06.019
Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN et al (2014) Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 71(2):327–349. https://doi.org/10.1016/j.jaad.2014.03.030
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K et al (2014) Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 71(1):116–132. https://doi.org/10.1016/j.jaad.2014.03.023
Paller AS, Beck LA (2022) Bleach baths for atopic dermatitis: Evidence of efficacy but more data are needed. Ann Allergy Asthma Immunol 128(6):617–618. https://doi.org/10.1016/j.anai.2022.03.013
Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS (2009) Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 123(5):e808–e814. https://doi.org/10.1542/peds.2008-2217
Gonzalez ME, Schaffer JV, Orlow SJ, Gao Z, Li H, Alekseyenko AV et al (2016) Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J Am Acad Dermatol 75(3):481-93e8. https://doi.org/10.1016/j.jaad.2016.04.066
Wong SM, Ng TG, Baba R (2013) Efficacy and safety of sodium hypochlorite (bleach) baths in patients with moderate to severe atopic dermatitis in Malaysia. J Dermatol 40(11):874–880. https://doi.org/10.1111/1346-8138.12265
Ryan C, Shaw RE, Cockerell CJ, Hand S, Ghali FE (2013) Novel sodium hypochlorite cleanser shows clinical response and excellent acceptability in the treatment of atopic dermatitis. Pediatr Dermatol 30(3):308–315. https://doi.org/10.1111/pde.12150
Khadka VD, Key FM, Romo-González C, Martínez-Gayosso A, Campos-Cabrera BL, Gerónimo-Gallegos A et al (2021) The skin microbiome of patients with atopic dermatitis normalizes gradually during treatment. Front Cell Infect Microbiol 11:910
Majewski S, Bhattacharya T, Asztalos M, Bohaty B, Durham KC, West DP et al (2019) Sodium hypochlorite body wash in the management of Staphylococcus aureus–colonized moderate-to-severe atopic dermatitis in infants, children, and adolescents. Pediatr Dermatol 36(4):442–447
Eriksson S, van der Plas MJA, Morgelin M, Sonesson A (2017) Antibacterial and antibiofilm effects of sodium hypochlorite against Staphylococcus aureus isolates derived from patients with atopic dermatitis. Br J Dermatol 177(2):513–521. https://doi.org/10.1111/bjd.15410
Sawada Y, Tong Y, Barangi M, Hata T, Williams MR, Nakatsuji T et al (2019) Dilute bleach baths used for treatment of atopic dermatitis are not antimicrobial in vitro. J Allergy Clin Immunol 143(5):1946–1948
Leung TH, Zhang LF, Wang J, Ning S, Knox SJ, Kim SK (2013) Topical hypochlorite ameliorates NF-κB–mediated skin diseases in mice. J Clin Investig 123(12):5361–5370
Shi V, Foolad N, Ornelas J, Hassoun L, Monico G, Takeda N et al (2016) Comparing the effect of bleach and water baths on skin barrier function in atopic dermatitis: a split-body randomized controlled trial. Br J Dermatol 175(1):212–214. https://doi.org/10.1111/bjd.14483
Hon KL, Tsang YC, Lee VW, Pong NH, Ha G, Lee ST et al (2016) Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate-to-severe eczema: a randomized, placebo-controlled cross-over trial. J Dermatolog Treat 27(2):156–162. https://doi.org/10.3109/09546634.2015.1067669
Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J (2012) EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 67(1):99–106. https://doi.org/10.1111/j.1398-9995.2011.02719.x
Thijs JL, Strickland I, Bruijnzeel-Koomen C, Nierkens S, Giovannone B, Csomor E et al (2017) Moving toward endotypes in atopic dermatitis: identification of patient clusters based on serum biomarker analysis. J Allergy Clin Immunol 140(3):730–737. https://doi.org/10.1016/j.jaci.2017.03.023
Akdis CA, Akdis M (2003) Immunological differences between intrinsic and extrinsic types of atopic dermatitis. Clin Exp Allergy 33(12):1618–1621. https://doi.org/10.1111/j.1365-2222.2003.01803.x
Kim DW, Park JY, Na GY, Lee SJ, Lee WJ (2006) Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int J Dermatol 45(6):698–701. https://doi.org/10.1111/j.1365-4632.2005.02644.x
Knor T, Meholjic-Fetahovic A, Mehmedagic A (2011) Stratum corneum hydration and skin surface pH in patients with atopic dermatitis. Acta Dermatovenerol Croat 19(4):242–247
Myer K, Maibach H (2013) Stratum corneum evaluation methods: overview. Skin Res Technol 19(3):213–219. https://doi.org/10.1111/srt.12011
Yoshida T, Beck LA, De Benedetto A (2022) Skin barrier defects in atopic dermatitis: from old idea to new opportunity. Allergol Int 71(1):3–13
Breternitz M, Flach M, Prassler J, Elsner P, Fluhr JW (2007) Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: integrity and cohesion assessed by sequential tape stripping. A randomized, controlled study. Br J Dermatol 156(2):231–240. https://doi.org/10.1111/j.1365-2133.2006.07632.x
Berthaud F, Boncheva M (2011) Correlation between the properties of the lipid matrix and the degrees of integrity and cohesion in healthy human Stratum corneum. Exp Dermatol 20(3):255–262. https://doi.org/10.1111/j.1600-0625.2010.01164.x
Bakaa L, Pernica JM, Couban RJ, Tackett KJ, Burkhart CN, Leins L et al (2022) Bleach baths for atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 128:660-668.e9
Mollanazar NK, Smith PK, Yosipovitch G (2016) Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol 51(3):263–292. https://doi.org/10.1007/s12016-015-8488-5
Chrostowska-Plak D, Reich A, Szepietowski JC (2013) Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol 27(2):e239–e242. https://doi.org/10.1111/j.1468-3083.2012.04578.x
Erickson S, Heul AV, Kim BS (2021) New and emerging treatments for inflammatory itch. Ann Allergy Asthma Immunol 126(1):13–20. https://doi.org/10.1016/j.anai.2020.05.028
Fukuyama T, Martel B, Linder K, Ehling S, Ganchingco J, Bäumer W (2018) Hypochlorous acid is antipruritic and anti-inflammatory in a mouse model of atopic dermatitis. Clin Exp Allergy 48(1):78–88
Kabashima K (2013) New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci 70(1):3–11. https://doi.org/10.1016/j.jdermsci.2013.02.001
Janssens M, van Smeden J, Puppels GJ, Lavrijsen AP, Caspers PJ, Bouwstra JA (2014) Lipid to protein ratio plays an important role in the skin barrier function in patients with atopic eczema. Br J Dermatol 170(6):1248–1255. https://doi.org/10.1111/bjd.12908
Elias PM (2014) Lipid abnormalities and lipid-based repair strategies in atopic dermatitis. Biochim Biophys Acta 1841(3):323–330. https://doi.org/10.1016/j.bbalip.2013.10.001
Yuki T, Tobiishi M, Kusaka-Kikushima A, Ota Y, Tokura Y (2016) Impaired tight junctions in atopic dermatitis skin and in a skin-equivalent model treated with interleukin-17. PLoS ONE 11(9):e0161759. https://doi.org/10.1371/journal.pone.0161759
De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C et al (2011) Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol 127(3):773-86e1-7. https://doi.org/10.1016/j.jaci.2010.10.018
Banovic F, Olivry T, Bäumer W, Paps J, Stahl J, Rogers A et al (2018) Diluted sodium hypochlorite (bleach) in dogs: antiseptic efficacy, local tolerability and in vitro effect on skin barrier function and inflammation. Vet Dermatol 29(1):6-e5
Wertheim HF, Verveer J, Boelens HA, van Belkum A, Verbrugh HA, Vos MC (2005) Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob Agents Chemother 49(4):1465–1467. https://doi.org/10.1128/AAC.49.4.1465-1467.2005
Davis MF, Iverson SA, Baron P, Vasse A, Silbergeld EK, Lautenbach E et al (2012) Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infect Dis 12(9):703–716. https://doi.org/10.1016/S1473-3099(12)70156-1
Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P et al (2020) IL-4Ralpha blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol 140(1):191-202e7. https://doi.org/10.1016/j.jid.2019.05.024
Leshem YA, Hajar T, Hanifin JM, Simpson EL (2015) What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 172(5):1353–1357. https://doi.org/10.1111/bjd.13662
Elman S, Hynan LS, Gabriel V, Mayo MJ (2010) The 5-D itch scale: a new measure of pruritus. Br J Dermatol 162(3):587–593. https://doi.org/10.1111/j.1365-2133.2009.09586.x
Desai NS, Poindexter GB, Monthrope YM, Bendeck SE, Swerlick RA, Chen SC (2008) A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol 59(2):234–244. https://doi.org/10.1016/j.jaad.2008.04.006
Fluhr JW, Feingold KR, Elias PM (2006) Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Exp Dermatol 15(7):483–492. https://doi.org/10.1111/j.1600-0625.2006.00437.x
Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM (2003) pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol 121(2):345–353. https://doi.org/10.1046/j.1523-1747.2003.12365.x
Oh J, Byrd AL, Deming C, Conlan S, Program NCS, Kong HH et al (2014) Biogeography and individuality shape function in the human skin metagenome. Nature 514(7520):59–64. https://doi.org/10.1038/nature13786
Acknowledgements
The authors would like to thank our clinical coordinators Dr. Diane Wang and Ms. Jean Sauvain in the Dermatology Clinical Trials Unit for their help. Dr. Suephy Chen (Chairman of the Department of Dermatology, Duke University Medical Center) provided us with her validated instrument developed to assess how chronic itch affects patient quality of life (ItchyQoLTM). Dr. Dwight Hardy and Mr. David Vicino from URMC Microbiology lab for determining S. aureus culture-positivity of swab samples. Ann Gill in Dr. Steve Gill’s laboratory for processing isolated DNA samples for 16S rRNA gene sequencing. Dr. Julie Ryan Wolf for her suggestions and advice on statistical analyses.
Funding
This work was supported by the National Institutes of Health [NIAMS grant number 5R21AR062357 (grant to LAB)]; the National Eczema Association (grant to NP-N)].
Author information
Authors and Affiliations
Contributions
Authors AS, NP-N, and SAK contributed equally to this work and are joint first authors. All authors were involved in the interpretation of data, drafting and reviewing the manuscript, and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report relevant to this manuscript.
Ethical approval
The final protocol, amendments, and informed consent documentation were reviewed and approved by the institutional review board of the University of Rochester Medical Center. All patients provided written informed consent. This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonization Good Clinical Practice Guidelines.
Consent to participate
Informed consent was obtained from all participants included in this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stolarczyk, A., Perez-Nazario, N., Knowlden, S.A. et al. Bleach baths enhance skin barrier, reduce itch but do not normalize skin dysbiosis in atopic dermatitis. Arch Dermatol Res 315, 2883–2892 (2023). https://doi.org/10.1007/s00403-023-02723-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-023-02723-1