Abstract
Patients with early-stage disease typically have a good prognosis, but still have a risk of recurrence, even with negative sentinel lymph node biopsy (SLNB). This study explores the utility of routine imaging to detect metastases in patients with negative SLNB but high-risk 31 gene expression profile (31-GEP) scores. We retrospectively identified melanoma patients with negative SLNBs. Patients with high-risk GEP results were placed in the experimental group and patients without GEP testing were placed in the control group. Among both cohorts, recurrent melanoma groups were identified. The tumor burden at the time of recurrence and the time to recurrence were compared between experimental group patients with routine imaging and control group patients without imaging schedules. We identified 327 control patients and 307 experimental patients, of which 14.1% versus 20.5% had melanoma recurrence, respectively. Of the patients with recurrent melanoma, those in the experimental group were older (65.75 versus 59.20), had higher Breslow depths (3.72 mm versus 3.31 mm), and had advanced tumor staging (89.5% versus 71.4% of patients presenting clinical stage ≥ II) compared to the control group at primary diagnosis. However, melanoma recurrence was detected earlier (25.50 months versus 35.35 months) in the experimental group at a lower overall tumor burden (73.10 mm versus 27.60 mm). A higher percentage of experimental patients started immunotherapy when offered (76.3% and 67.9%). Patients who received routine imaging after high-risk GEP test scores had an earlier recurrence diagnosis with lower tumor burden, leading to better clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunotherapies such as anti-programmed cell death ligand 1(anti-PD-1) immune checkpoint inhibitor (ICI) and anti-cytotoxic T lymphocyte antigen-2 (anti-CTLA-4) ICI, as well as targeted antitumor treatments, including B-Raf proto-oncogene (BRAF) and mitogen-activated protein-kinase-kinase (MEK) inhibitors, have revolutionized melanoma treatment [1, 2]. Follow-up data support the effectiveness of these newer therapies in improving progression-free survival (PFS) and overall survival (OS) [1,2,3]. Importantly, many trials involving these novel agents suggest greater efficacy when administered to patients with an initial lower tumor burden [4,5,6,7,8].
Routine imaging can effectively detect early relapse when there is a lower tumor burden [9,10,11]. For patients with stage IIB disease or greater, the National Comprehensive Cancer Network® (NCCN®) recommends chest radiography, CT (CT), brain magnetic resonance imaging (MRI), and positron emission tomography and CT (PET/CT) every 3–12 months for 3 years at the discretion of the physician [12]. However, routine imaging is not a standard protocol for clinical stage 1 and 2 patients, and the guidelines for surveillance remain controversial.
Notably, recurrence in patients with early-stage disease is well documented and may be as high as 40–69% [12,13,14]. Additionally, AJCC and SEER data show that, excluding stage IV patients, 60% of patients who ultimately die from metastatic melanoma are stage 1 or 2 at the time of initial diagnosis [15,16,17]. Hence, a significant number of patients diagnosed with early-stage disease may have aggressive melanomas that may recur and ultimately result in death. Any method of determining which patients may most benefit from routine imaging should aim at identifying patients at a point of low total tumor burden, as they may have the greatest chance for cure or improved survival with immunotherapy or targeted therapy.
A 31-gene expression profile (31-GEP) test was introduced in 2013 and yields a continuous probability score between zero and one that stratifies the risk of melanoma disease recurrence. The score is assigned to four categories: low risk of recurrence (Class 1A; 0–0.41 and Class 1B; 0.42–0.49), and high risk of recurrence (Class 2A; 0.50–0.58 and Class 2B; 0.59–1) [18,19,20,21]. The 31-GEP Class has been demonstrated to be an independent predictor of recurrence, including nodal recurrence and distant metastasis-free survival (DMFS) in meta-analyses and multiple prospective and retrospective studies [20, 22]. This study looked at patients with a negative sentinel lymph node (SLN) biopsy and a high-risk 31-GEP result. We then selected patients with recurrence and compared the tumor burden between patients who underwent a routine imaging protocol to those who did not. Specifically, we compared our experimental cohort with a control cohort of patients with negative SLN biopsy results who did not have GEP testing and only had imaging studies as indicated by clinical symptoms to validate the utility of GEP results in guiding radiological surveillance. Patients with high-risk recurrence scores from GEP testing who were subsequently placed on routine imaging had an earlier recurrence diagnosis with lower tumor burden with a trend towards improved overall survival.
Methods
Study design and patient selection
Retrospective chart reviews were performed at Northwestern University, Cleveland Clinic, and Oregon Health & Science University. All patients with a confirmed melanoma diagnosis and a negative sentinel lymph node biopsy (pathologic Stage 1 or 2 disease at the initial diagnosis) were selected for this study (Fig. 1). Patients tested with a 31-GEP (Castle Biosciences, Friendswood, TX) and with a GEP Class result of 2A or 2B were recruited into the experimental group, while Class 1A and 1B patients were excluded from the study. Patients who were Class 2A/B but without a SLNB were excluded from this study. All patients without GEP testing were placed in the control group if scheduled routine imaging was not part of the follow-up plan. In this control group, imaging studies were driven by symptoms or physical exam findings. Within each group, subgroups were identified as patients with visceral or lymphatic melanoma recurrence versus those without recurrence. Patients with routine imaging in the melanoma recurrent control group or those who did not adhere to imaging schedules in the melanoma recurrent experimental group were excluded from the melanoma recurrence groups.
Tumor burden at the first date of detection of recurrence was compared between the experimental subgroup with recurrence versus the control subgroup with recurrence. Patients were only included in the experimental subgroup of recurrent disease patients if they adhered to routine imaging schedules and had metastasis detection because of routine imaging rather than imaging performed as a result of a clinical symptom. The first endpoint was the date of detection of the first evidence of recurrence and the date of the last chart review was the secondary endpoint. The primary outcome of this study was the total tumor burden calculated at the first identified time of any evident recurrence of melanoma. Although there were differences between sites and patients, the routine imaging protocol typically consisted of chest CT without contrast, abdominal pelvic CT with contrast, and brain MRIs with and without contrast at an average of 6-month intervals.
Tumor burden measurement, treatment outcomes, and survival analysis
Imaging reports interpreted by attending radiologists were used to determine tumor burden. The first radiology report in which the diagnosis of metastatic melanoma was suspicious enough to provide a measurement of the tumor was used to determine the initial tumor burden and the date of the first recurrence. Hence, the first sign of measurable tumor burden was used as the primary endpoint. If multiple foci were identified, the measurements were added together to determine the total tumor burden. Additional foci identified in subsequent studies that were not present in the first imaging study were not included in the measurement. All imaging studies that were part of the initial workup identifying measurable tumor burden were included. The tumor burden measurement was calculated using an adapted version of the response evaluation criteria in solid tumors (RECIST) 1.1 criteria by calculating the unidimensional sum of all reported metastatic foci present in initial imaging studies (method of Dall’Olio et al. [23, 24]. Specifically, the measured sums of the longest single length of all included metastatic foci led to the total tumor burden. Unlike the RECIST criteria, which evaluate the change in tumor burden, no tumor exclusions were made based on the minimum tumor size or the maximum number of tumors present in any organ, and ultrasound (US) examination measurements were included to determine the initial metastatic foci present that were ultimately included in tumor burden measurement [24].
Patient charts were reviewed to determine if they were treated with immunotherapy or other agents after the first detection of melanoma recurrence. Patient survival data was determined using the second study endpoint, which was the date of the last review of the chart.
Analysis
The tumor burden differences between the control and experimental groups were evaluated using a two-sample t-test with unequal variances, and a two-sided P value < 0.05 was considered statistically significant. The time to progression was from the diagnosis of primary melanoma to detection of visceral or lymphatic metastases. Kaplan–Meier estimates with the log-rank test analysis were used to assess months melanoma recurrence between the experimental and control groups. Descriptive statistics included sex, age, average Breslow depth, tumor staging, and the site of initial melanoma and recurrence. The Chi-Square and Kruskal–Wallis statistic were used to determine the significance between both groups among each descriptive variable. P values ≤ 0.05 were considered significant. Statistical analysis was performed using Microsoft Excel and XLSTAT 2022.
Results
A total of 307 patients with stage 1 or 2 clinical disease and a GEP Class 2A/B result were included in the experimental group. In comparison, 327 stage 1 or 2 patients without GEP testing were included in the control group. There were 63 recurrences in the experimental group versus 46 in the control group, which was statistically significant (20.5% versus 14.1%, p-value 0.031). Among the 63 recurrences in the experimental group, 38 patients followed a routine imaging protocol, while 25 did not, so they were excluded from the primary endpoint analysis for tumor burden. None of the patients in the control group followed an imaging protocol.
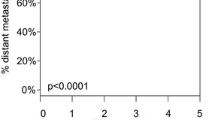
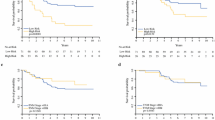
The average tumor burden among recurrent melanoma patients in the experimental group was 27.60 mm compared to 73.10 mm in recurrent melanoma patients from the control group (Fig. 2), which was statistically significant with a p-value of 0.027. Kaplan–Meier analysis (Fig. 3) also showed that the time difference between the detection of melanoma recurrence in the experimental group and control group was significant; on average, melanoma recurrence was detected at 25.50 months in the experimental group versus 35.35 months for the control group (p-value of 0.049; t-test p-value of 0.004).
Among patients with recurrent melanoma, the experimental cohort had a greater average Breslow depth (3.72 mm vs. 3.31 mm), older age (65.75 years vs. 59.2 years) at primary diagnosis, and a higher proportion of clinical stage 2 patients (89.5% vs. 71.4%) compared to the control group. None of these findings met statistical significance (Table 1). There were also no significant differences regarding gender, initial tumor site, and distant recurrences to brain, lymph nodes, and total visceral recurrences. Lungs were the most common distant metastatic site, present in 65.2% of all melanoma recurrent patients, followed by lymph nodes with 31.8%.
The percentage of patients who started immunotherapy was 81.6% (31/38) among recurrent melanoma patients in the experimental group and 71.4% (20/28) among recurrent melanoma patients in the control group. At the time of the last follow-up, 76.3% (29/38) of the patients with melanoma recurrence in the experimental group were alive with an average follow-up time of 45.63 months, compared to 50.0% (14/28) of recurrent melanoma patients in the control group with an average follow-up time of 63.32 months. The difference in overall survival was statistically significant, with a chi-square p-value of 0.027.
Discussion
Historically, early detection of metastatic disease was considered unjustified, given the lack of effective treatment options prior to the recent advances in of systemic therapies [13]. However, recent studies suggest a survival benefit when metastatic melanoma is treated at a lower tumor burden [4,5,6,7,8]. The COMBI-d and COMBI-v trials showed a significant improvement in OS and PFS in patients treated with dabrafenib plus trametinib (MEK inhibitor) when patients started at less than 3 tumor sites (n = 282) versus more than or equal to 3 tumor sites (n = 269) [25]. Likewise, in studies of patients treated with the anti-PD-1 antibody, pembrolizumab, OS decreased as the number of metastatic lesions increased, and patients with a longer PFS generally had a lower tumor burden [6, 26]
In a retrospective medical imaging review of 10 patients with metastatic melanoma treated with dabrafenib, the mortality hazard tripled for every 1 cc increase in tumor volume (p = 0.047, HR 2.81, CI 1.06 –6.19), and patients with tumor volumes above the median of 111.5 cc also had a statistically significant shorter OS than patients with smaller tumor volumes (6 months vs. 56 months, p-value = 0.019) [27].
In contrast to using tumor volumes or sites, our study used cumulative tumor size to measure tumor burden. We calculated the clinical tumor burden as the sum of the largest unidimensional lengths of all metastatic foci. Specifically, we compared the tumor burden at the time of the first evidence of melanoma recurrence in a cohort of patients with stage 1 or 2 disease and with GEP Class 2A/B accompanied by routine imaging with a cohort of patients with stage 1 or 2 disease melanoma without GEP testing or routine imaging. Despite having a higher mean Breslow and a higher proportion of patients with clinical stage II disease or greater, the experimental group had a significantly lower tumor burden detected at the first recurrence (27.60 mm verse 73.10 mm).
As a secondary endpoint we assessed overall survival at the time of last follow up. In accordance with the higher rate of recurrence in the experimental group, more patients with recurrent melanoma in the experimental group were started on immunotherapy. Importantly, patients in the experimental group had statistically significant better overall survival (76.3% versus 50.0%). It is important to note that there was a difference in average follow-up times between the control and experimental groups, with an average follow-up of 63.32-months and 45.63 months, respectively. Given that the follow-up time was longer in the control group, this may have led to more deaths reported. Therefore, we analyzed both melanoma recurrent experimental and control group patients at the 45.63-month mark, which showed a similar trend in results, though not significant. Specifically, at a maximum follow-up of 45.63 months, 86.80% of the melanoma recurrent experimental patients were alive, and 75.00% of the melanoma recurrent control group patients were alive. A trend towards improved overall survival in patients with recurrent melanoma of the experimental group supports previous studies suggesting that response to newer systemic therapies may be better when tumor burden is lower [4,5,6,7]. Hence, in the current era of novel targeted and immunotherapy there may be a need for greater emphasis on detecting metastatic disease earlier.
Past studies evaluating the impact of imaging studies on early detection of recurrent diseases have had mixed results. This may be related to large sample sizes that included patients with minimal risk of metastasis [28]. As expected, when implementing an interventional strategy in cohorts of patients with minimal risk of metastasis, the odds of finding a statistically significant benefit are low. [29, 30]. However, more are recent studies suggest that in higher risk patients, routine imaging can identify early visceral or lymphatic melanoma metastasis in clinically asymptomatic patients [13, 31]. In our study, we found that surveillance imaging detected melanoma recurrence 9.84 months earlier (25.50 months vs. 35.35 months) in patients who had routine imaging schedules compared to those who did not. This included visceral (92.1%), nodal (36.8%) and CNS (15.8%) recurrences. Therefore, surveillance imaging can detect melanoma recurrence in high-risk patients at an earlier time frame with a lower overall tumor burden.
Compared to routine clinical exams, imaging studies are more costly and should be used strategically according to the patient's risk of recurrence [9]. In fact, NCCN guidelines recommend that follow-up of patients be based on their level of risk of relapse (13). The 31-GEP test is a tool for identifying the risk of melanoma relapse, which has been particularly shown to identify a high risk of recurrence in patients with clinical stage 1 or 2 AJCC disease [32]. In a study of 259 patients with negative SLNBx, 70% of patients with high risk of recurrence (Class 2) 31-GEP results experienced metastasis [32]. In our study of patients with stage 1 and 2 disease, significantly more patients with high score 31-GEP results experienced melanoma recurrence compared to those without 31-GEP testing (20.5% vs. 14.1%). Therefore, the 31-GEP tool may offer one strategy of identifying high risk stage 1 and 2 patients who ultimately account for a significant proportion of melanoma related deaths.
The limitations of our study included the retrospective nature of this study, with a limited sample size of patients with recurrent melanoma. In addition, there were less than uniform imaging protocols among all three sites, including the type of imaging study recommended. There was a difference in the durations of the surveillance intervals, which ranged from 6 to 12 months. Furthermore, among all three sites and both groups, there was also a difference in the patient follow-up lengths for patients with recurrence.
In summary, the 31-GEP test identifies patients who are at a higher risk of developing metastases, and when combined with routine imaging studies, patients with visceral or lymphatic metastases can be identified and offered systemic and immunotherapy treatment in an earlier time frame with a lower tumor burden, which can lead to improved patient outcomes.
Data availability
The data generated in this study are not publicly available but deidentified data are available upon reasonable request from the corresponding author.
Change history
12 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00403-023-02622-5
25 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00403-023-02733-z
References
Namikawa K, Yamazaki N (2019) Targeted Therapy and Immunotherapy for Melanoma in Japan. Curr Treat Options Oncol 20(1):7. https://doi.org/10.1007/s11864-019-0607-8. (in English)
Weiss SA, Wolchok JD, Sznol M (2019) Immunotherapy of melanoma: facts and hopes. Clin Cancer Res 25(17):5191–5201. https://doi.org/10.1158/1078-0432.Ccr-18-1550. (in English)
Lugowska I, Teterycz P, Rutkowski P (2018) Immunotherapy of melanoma. Contemp Oncol (Pozn) 22(1a):61–67. https://doi.org/10.5114/wo.2018.73889. (in English)
Poklepovic AS, Carvajal RD (2018) Prognostic value of low tumor burden in patients with melanoma. Oncology (Williston Park) 32(9):e90–e96 (in English)
Ribas A et al (2016) Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315(15):1600–1609. https://doi.org/10.1001/jama.2016.4059. (in English)
Huang AC et al (2017) T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545(7652):60–65. https://doi.org/10.1038/nature22079. (in English)
Meckbach D et al (2014) Survival according to BRAF-V600 tumor mutations–an analysis of 437 patients with primary melanoma. PLoS ONE 9(1):e86194
Long GV et al (2017) Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 28(7):1631–1639. https://doi.org/10.1093/annonc/mdx176. (in English)
Podlipnik S et al (2019) Cost-effectiveness analysis of imaging strategy for an intensive follow-up of patients with American Joint Committee on Cancer stage IIB, IIC and III malignant melanoma. Br J Dermatol 180(5):1190–1197. https://doi.org/10.1111/bjd.16833. (in English)
Park TS et al (2017) Routine computer tomography imaging for the detection of recurrences in high-risk melanoma patients. Ann Surg Oncol 24(4):947–951
Livingstone E et al (2015) Prospective evaluation of follow-up in melanoma patients in Germany: results of a multicentre and longitudinal study. Eur J Cancer 51(5):653–667. https://doi.org/10.1016/j.ejca.2015.01.007
N. C. C. Network (2022) Melanoma: cutaneous (Version 3.2022). https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed 31 Oct 2021
Freeman M, Laks S (2019) Surveillance imaging for metastasis in high-risk melanoma: importance in individualized patient care and survivorship. Melanoma Manag 6(1):12
Tas F (2012) Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors,". Journal of oncology. https://doi.org/10.1155/2012/647684
Whiteman DC, Baade PD, Olsen CM (2015) More people die from thin melanomas (≤ 1 mm) than from thick melanomas (>4 mm) in Queensland, Australia. J Invest Dermatol 135(4):1190–1193. https://doi.org/10.1038/jid.2014.452. (in English)
Balch CM et al (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27(36):6199–6206. https://doi.org/10.1200/jco.2009.23.4799
Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC, Halpern AC (2016) Melanoma thickness and survival trends in the United States, 1989–2009. J Natl Cancer Institute. https://doi.org/10.1093/jnci/djv294
Gerami P et al (2015) Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res 21(1):175–183. https://doi.org/10.1158/1078-0432.Ccr-13-3316. (in English)
Gerami P et al (2015) Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J Am Acad Dermatol 72(5):780–5.e3. https://doi.org/10.1016/j.jaad.2015.01.009. (in English)
Zager JS et al (2018) Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer 18(1):130. https://doi.org/10.1186/s12885-018-4016-3. (in English)
Whitman ED et al (2021) Integrating 31-gene expression profiling with clinicopathologic features to optimize cutaneous melanoma sentinel lymph node metastasis prediction. JCO Precis Oncol. https://doi.org/10.1200/po.21.00162. (in English)
Jarell A et al (2021) The 31-gene expression profile stratifies recurrence and metastasis risk in patients with cutaneous melanomax. Future Oncol 17(36):5023–5031. https://doi.org/10.2217/fon-2021-0996. (in English)
Dall’Olio FG et al (2022) Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol 19(2):75–90. https://doi.org/10.1038/s41571-021-00564-3. (in English)
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026. (in English)
Robert C et al (2019) Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381(7):626–636. https://doi.org/10.1056/NEJMoa1904059. (in English)
Kim J et al (2022) A comparison of 2 disease burden assessment methods (3D volume vs the number of lesions) for prognostication of survival in metastatic melanoma: implications for the characterization of oligometastatic disease. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2022.08.040. (in English)
Ali A et al (2020) Correlation between initial tumour volume and treatment duration on Dabrafenib: observation study of subjects with BRAF mutant melanoma on the BRF112680 trial. BMC Cancer 20(1):342. https://doi.org/10.1186/s12885-020-06848-8. (in English)
Pozsgai M, Németh K, Oláh P, Gyulai R, Lengyel Z (2021) The significance of imaging examinations during follow-up for malignant melanoma. Eur J Dermatol 31(3):357–363. https://doi.org/10.1684/ejd.2021.4054. (in English)
Baker JJ, Meyers MO, Frank J, Amos KD, Stitzenberg KB, Ollila DW (2014) Routine restaging PET/CT and detection of initial recurrence in sentinel lymph node positive stage III melanoma. Am J Surg 207(4):549–554. https://doi.org/10.1016/j.amjsurg.2013.04.012. (in English)
Horn J, Lock-Andersen J, Sjøstrand H, Loft A (2006) Routine use of FDG-PET scans in melanoma patients with positive sentinel node biopsy. Eur J Nucl Med Mol Imaging 33(8):887–892. https://doi.org/10.1007/s00259-006-0077-7. (in English)
El-Shourbagy KH, Mashaly EM, Khodair SA, Houseni MM, Abou Khadrah RS (2020) PET/CT in restaging, prognosis, and recurrence in patients with malignant melanoma. Egypt J Radiol Nucl Med 51(1):167. https://doi.org/10.1186/s43055-020-00276-1
Gastman BR, Gerami P, Kurley SJ, Cook RW, Leachman S, Vetto JT (2019) Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol 80(1):149-157.e4. https://doi.org/10.1016/j.jaad.2018.07.028. (in English)
Funding
This work was supported by the IDP Foundation, Inc.
Author information
Authors and Affiliations
Contributions
S.D. collected and analyzed data, wrote the main manuscript text, prepared table 1, and prepared figures 1-3. D.B. and G.F. collected and assisted in analyzing data and writing the main manuscript text. M.H, N.L, S.O., M.F., and K.V. all edited manuscript text and reviewed figures. J.W., B.G., J.V., and P.G., all prepared the study design and conceptualization, as well as performed through manuscript review and edits. All authors reviewed the manuscript, figures 1-3, and Table 1.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Pedram Gerami, Dr. John Vetto, and Dr. Jeffery D. Wayne, have severed as consultants for Castle Biosciences, Inc. and received honoraria for consultant work. No other authors have conflicts of interest to disclose. This study was performed independent of Castle Biosciences, Inc, and was not supported or funded by them in any manner.
Consent to participate/consent to publish
This study was reviewed by the Institutional Review Board and given the retrospective chart review design it was determined that it involves only minimal risk to human subjects. A Waiver of HIPPA Authorization was granted to permit use of deidentified health information for research purposes.
Clinical trial registration
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective open access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dhillon, S., Duarte-Bateman, D., Fowler, G. et al. Routine imaging guided by a 31-gene expression profile assay results in earlier detection of melanoma with decreased metastatic tumor burden compared to patients without surveillance imaging studies. Arch Dermatol Res 315, 2295–2302 (2023). https://doi.org/10.1007/s00403-023-02613-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-023-02613-6