Abstract
Various therapeutic options are available for verruca. While physical destruction may be associated with scarring, immunotherapy may be effective in treating warts through stimulating body immune response. The objective of the study was to compare the efficacy, safety, and outcome of Candida antigen vs diphencyprone (DPCP) in the treatment of warts. Fifty patients were randomly assigned to receive either intralesional Candida antigen every 3 weeks or weekly DPCP application. Both treatments were applied only to the mother wart. Lesions’ clearance and associated side effects were observed up to 4 weeks after treatment. Two blinded physicians evaluated photos of warts before and 4 weeks after the end of treatment. Both modalities granted wart clearance and/or improvement with no statistically significant difference; however, Candida antigen was significantly better in clearing adjacent untreated warts (p = 0.046). Fewer side effects were observed among the Candida antigen group. The response was duration associated in the Candida groups only. Intralesional Candida antigen injection and DPCP treatments for warts yielded improvement with superiority of Candida injection in eradicating distant lesions and fewer side effects. A shorter wart duration may be associated with a better therapeutic response with Candida antigen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Human papilloma virus (HPV)-induced verruca are benign proliferation that may occur at any part of the skin. [1] Verruca treatment includes topical keratolytics, cryotherapy, electrocautery, chemical cautery, and laser ablation. [2] Such modalities can be painful and time consuming, and none of them is considered as gold standard due to potential scarring, disfigurement, and recurrence.
The mechanism of action of immunotherapy in wart treatment is not yet clear; however, it may boost the immune system to recognize the antigen via the delayed hypersensitivity reaction and subsequently clear the HPV [3]. Hence, it also has a potential to resolve distant warts, possibly by proliferation of HPV-specific mononuclear cells [5].
Candida was the first injectable antigen to be reported as a successful option in the treatment of warts [6]. Topical immunotherapy with diphenylcyclopropenone (DPCP) has also showed efficacy in topically treating recalcitrant warts [7]. In the present study, we compared two different modalities of immunotherapy, namely intralesional Candida antigen versus contact DPCP in terms of efficacy and safety for the treatment of verruca.
Materials and methods
This study was conducted between July 2019 and June 2020 at the outpatient clinic of the dermatology department of Badr University Hospital, Helwan University. The study protocol was approved by the Faculty of Medicine, Helwan University Research Ethics Committee. All the participants received full information prior to enrollment and an informed consent was obtained. Fifty patients were recruited, either with recalcitrant or non-recalcitrant warts. Warts were considered recalcitrant, when they failed to clear following treatment with two or more different modalities. We excluded patients receiving any immune-altering drugs (e.g., systemic steroids, chemotherapy) as well as those with a history of any disorder affecting their immune system, e.g., HIV and diabetes, pregnant and lactating females, patients with atopic dermatitis or history of other allergic disorders, and patients with history of wart treatment within 12 weeks prior to enrollment were also excluded.
During the first visit following recruitment, full history was obtained, thorough clinical examination was performed, and warts’ lesions were photographed with detailed data documentation. Participants were randomly assigned to group A or B using the sealed envelope technique.
Group A (candida antigen group)
Before inclusion into this group, patients were injected with 0.1 ml (ml) of 1/1000 purified Candida antigen solution (manufacturer: Ain Shams University Hospital, Specific Desensitizing Vaccine unit; manufacture date: March 2018, expiry date: March 2021) intradermally into the flexor aspect of the forearm using 1 ml insulin syringes. After 48–72 h, the existence of a visible cutaneous reaction in the form of erythema and induration of at least 5 mm in diameter was considered as positive sensitization response to the Candida antigen. All the enrolled patients showed a positive response to the Candida antigen and proceeded to the next step of starting active therapy. All recruits in this group were then injected with 0.1 ml of the solution in only the mother wart (the earliest or initial wart on the body). If the patient was not sure about the initial wart, then the largest wart was injected. Injections were administered at 3-week intervals until either complete clearance or a maximum of three treatment sessions. The same wart was injected at all visits in every patient.
Group B (DPCP group)
In this group, all the patients were subjected to DPCP solution (manufacture date: Feb. 2018, expiry date: Feb. 2021, Batch No. B22018, origin: Merck KGaA) at a concentration of 0.1% applied to the upper inner arm to induce sensitization using a cotton-tipped applicator. The application site was examined the following week for any eczematous reaction. Once the patient was sensitized, DPCP was applied to the mother wart with concentration starting from 0.1% and gradually increased (up to 2%) until a mild eczematous reaction was noticed. Application was performed weekly until the lesion cleared or completion of a maximum of five treatment sessions. The concentration of DPCP was adjusted according to the severity of the inflammatory reaction from the previous application.
Evaluation
All patients were evaluated at the beginning of the study and at each follow-up visit. The final response was assessed after 4 weeks of the last session for any signs of clearance of the treated and adjacent warts and all complications were recorded. Moreover, two blinded dermatologists were asked to evaluate the response by scoring photos of treated lesions before therapy and 4 weeks following the last session. The observers were asked to score the degree of improvement in number and size of treated and adjacent untreated warts as follows: 0 = no change, 1 = less than 25% improvement, 2 = 25–50% improvement, 3 = 51–75% improvement and 4 = 76–100% improvement. Patient scores of 0 or 1 were considered as unresponsive.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (± SD), median and range, or frequencies (number of cases) and percentages when appropriate. Numerical data were tested for the normal assumption using Shapiro–Wilk test. Comparison of numerical variables between the study groups was done using Mann–Whitney U test for independent samples. For comparing categorical data, Chi-square (χ2) test was performed. Exact test was used instead when the expected frequency was less than 5. Correlation between the different variables was done using Spearman equation. Accuracy was represented using the terms sensitivity, specificity, + ve predictive value, –ve predictive value, and overall accuracy. Two-sided p values less than 0.05 were considered statistically significant. All statistical calculations were done using computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, USA) release 22 for Microsoft Windows.
Results
Forty patients completed the study, while 10 patients dropped due to irritability from the sensitization reaction induced by Candida antigen and/or DPCP at the start of therapy. There was no significant difference between the two groups regarding participants’ demographic and clinical data including wart type, duration, or recalcitrance (Table 1).
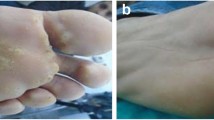
For all recruits, 17/40 (42.5%) patients showed complete clearance of the central treated wart, with 12/20 (60%) patients in the Candida-treated group (Figs. 1, 2, supplementary Figs. 1, 2, 3), and 5/20 (25%) patients in the DPCP-treated group (Figs. 3, 4; supplementary Figs. 4, 5, 6), but this was not statistically significant (95% CI 1.037–5.555, p = 0.054). Similarly, there was no significant difference in observers’ evaluation scores for improvement between the Candida antigen and DPCP groups (p = 0. 580). Twenty-six recruits had warts adjacent to the treated mother wart. Only 3/10 (30%) patients in the Candida-treated group and 0/16 patients in the DPCP antigen-treated group showed clearance of their adjacent warts and this was statistically significant (p = 0.046) (Table 1).
For all recruits, there was no statistical difference in treatment outcomes as assessed by the median observers’ score for improvement between cases with plantar warts and those with other types of warts (p = 0.385).
Relation between duration and treatment outcomes ( Table 2 )
For the Candida antigen treatment group, patients with shorter wart duration showed a significantly higher clearance rate of mother and adjacent warts (p = 0.001 and 0.022, respectively), while this was not observed in the DPCP contact therapy group. Similarly, non-responders (less than 25% improvement according to observers’ scores for improvement) had significantly longer disease duration than responding patients in the Candida antigen-treated group (p = 0.021), but not within the DPCP-treated group (p = 0.612).
Moreover, a significant negative association between the number of sessions and observers’ score for improvement was detected in the Candida antigen treatment group (r = – 0.605, p = 0.005), but not in the DPCP treatment group (r = – 0.090, p = 0.707).
Recalcitrant warts showed a significantly longer duration (p = 0.000), worse observers’ scores for improvement (p = 0.018) and less post-intervention tenderness (p = 0.009) in comparison to non-recalcitrant warts. There was no significant difference in improvement scores between recalcitrant and non-recalcitrant warts within neither the Candida nor the DPCP-treated groups (p = 1.00 and 0.260 respectively). No statistical difference in observers’ improvement scores for recalcitrant warts could be detected between Candida antigen and DPCP treatment groups (p = 0.678).
Side effects (supplementary Tables 1,2)
Eight patients (40%) in the Candida antigen group suffered side effects of treatment (supplementary Fig. 7) in comparison to 20 patients (100%) in the DPCP group (supplementary Fig. 8) and this difference was statistically significant (p = 0.002). Redness and vesicle formation were significantly associated with DPCP treatment in comparison to Candida antigen (p = 0.013 and 0.004, respectively). A significant positive association between the number of sessions and treatment-induced tenderness and redness was observed in the DPCP group only (p = 0.012 and 0.035, respectively). Except for ten patients who refused to continue after sensitization (not included in the analysis), side effects in both groups were expected, tolerable, transient and did not necessitate stoppage of treatment in any of the studied patients.
Discussion
HPV-associated verrucas still pose a therapeutic dilemma because of the possible associated disfigurement, recurrence, or inefficiency of the treatment options available [8]. In the current study, verruca treatment with Candida antigen injection or topical DPCP contact therapy resulted in clearance of the treated lesion in 42% of patients with no permanent adverse effects or scarring. Moreover, treatment with the Candida antigen was associated with clearing of untreated adjacent warts in 30% of patients.
Immunotherapy for warts seems to alert the immune system about the presence of an antigen that requires an immune reply with a consequent type IV delayed-type hypersensitivity response along with upregulation of IL-1, IL-12, TNF-α, and IFN-γ. This immune response, if elicited, is not specific to the injected antigen, but may also act against wart tissue [9]. Moreover, contact immunotherapy was shown to be associated with a better response in warts of patients treated with squaric acid dibutyl ester (SADBE), specifically IL-4 and IL-10 [9, 10]. Similarly, IL-10 was also reduced in warts of patients treated successfully with PPD [9]. Whether injectable or contact therapy, immunotherapy for warts may exert its therapeutic response through upregulation of IL-1, IFN -γ, and TNF-α and downregulation of IL-4 and IL-10. [9, 11]
Previous studies suggest that intralesional antigen immunotherapy with Candida antigen may induce a nonspecific Th1 inflammatory reaction against HPV, as well as an associated specific Th1 response to papilloma virus capsid protein L1 [12,13,14,15]. Macrophage migration inhibitory factor (MIF) was shown to be upregulated among those with warts responding to Candida antigen injection [16]. Comparatively, topical immunotherapy with contact sensitizers including DPCP is believed to induce a delayed-type hypersensitivity reaction with the production of several cytokines that may stimulate natural killer cells toward wart tissue [17,18,19,20].
Our results show a higher clearance rate and a better observers’ scores for improvement in warts treated with the Candida antigen vs DPCP. Indeed, 60% of patients in the Candida antigen group showed complete clearance of central treated wart, in contrast to 25% of patients in the DPCP group; however, these differences were statistically insignificant. Similarly, the mean observers’ scores for improvement were better for the Candida antigen treatment group, but in a statistically insignificant manner. While 30% of our patients showed resolution of untreated warts with Candida antigen, this did not occur with DPCP. A larger number of patients may have showed more significant differences.
Similar to our findings, Khurshid et al. 2009 reported that Candida antigen was effective in clearing more than 60% of each injected wart in 67% of patients [21]. Other investigators reported that 56–81% of patients showed clearance of treated warts and 56–100% resolution of untreated warts with Candida antigen injections [5, 14, 22]. On the other hand, DPCP was previously shown to successfully clear warts in 66.6–82.9% of patients and in 100% of three children with anogenital warts when applied topically to each wart [23, 24]. Except for Van der Steen et al. 1999, no other investigators reported the effect of treatment with DPCP on untreated warts. The latter authors described a case of verruca plantaris that cleared with DPCP treatment associated with “some involution” in untreated warts. [25]
The demographic characteristics of recruited patients including age did not affect the therapeutic response with Candida or DPCP. This was in concordance with previous studies with Candida antigen [26]; however, Suh et al. reported that the therapeutic efficacy of DPCP gradually decreased with age [20]. The difference in mean age of recruited patients in the latter study in comparison to ours (14.9 Vs 30.1 years respectively) may account for such variance. It may also be related to varying levels of natural immune responses of different age groups, which may partially explain the low therapeutic efficacy of DPCP we encountered among our patients in comparison to the latter authors.
Based on our results, a significant association was observed between wart duration and the therapeutic response in the Candida group. This was in accordance with other studies that revealed that there is a significant inverse relationship between the disease duration and therapeutic response [26, 27].
While our result revealed that there was no significant association between wart duration and therapeutic response with DPCP immunotherapy, conversely another study revealed a significant decrease in success rate as disease duration increased, probably because of its lower capacity to stimulate the immune system in patients with long wart duration [28]. This contradiction may be attributed to different study settings where the aforementioned study examined only periungual warts, which is considered as a relative recalcitrance factor.
Patients with recalcitrant warts showed a significantly lower median observers’ score for improvement in comparison to those with non-recalcitrant lesions. Contrastingly, Garza et al. 2015 reported that in their cohort with 80.4% patients suffering from recalcitrant warts, complete clearance was achieved with Candida treatment in 70.9%; however, the study included only pediatric age group with a more robust immune response [29]. Hammad and coworkers also did not find an association between recalcitrance and response to Candida antigen injection, and suggested that failure to respond to Candida injection treatment may be due to elevated complement C3c and TNF-α in sera of non-responding patients [30]. The fact that recalcitrant warts showed significantly lower post-intervention tenderness than their non-recalcitrant counterparts in our cohort may suggest a frail immune response in these patients.
Our results showed that 40% of the Candida antigen group suffered side effects of treatment in comparison to 100% in the DPCP group and this difference was statistically significant (p value = 0.002). The side effects of both groups were tolerable, transient, and reversible, yet ten patients dropped out because of these; the findings agreed with previous studies [18, 26, 31,32,33,34,35]. As DPCP side effects tend to be more severe in highly sensitized individuals, [18, 35], it is advisable that patients showing severe local reactions on sensitization be closely monitored for adverse events.
Although, to the best of our knowledge, no comparisons between these two modalities could be retrieved, Candida antigen previously showed efficacy over isotretinoin, combined Candida and isotretinoin [36], photodynamic therapy [37] and 5 flurouracil [38] in the treatment of warts. Other immunotherapeutic agents showed similar results when compared to Candida such as combined bivalent human papillomavirus vaccine, [39] measles, mumps and rubella vaccine [40], purified protein derivative [41], herpes zoster vaccine [42] and tuberculin [43], while bleomycin [38], vitamin D [27, 44], cryo-immunotherapy [45] as well as alternating intralesional PPD and Candida [46] were superior to Candida.
The discrepancy in results among studies, including ours, for both Candida antigen and DPCP in the treatment of warts may be related to several factors. Differences in the preparations used, concentrations of antigen/DPCP, vehicle used, frequency of application, age of patients, site and duration of warts, state of recalcitrance and immune response variations among different ethnicities are among the main factors that can be responsible for such discrepancies.
The proper choice of treatment modality for warts has been discussed before [47, 48]. Our hypothesis is that different immunotherapeutic agents might vary in efficacy according to different patient and wart criteria. Hence, we believe designing comparative studies might help physicians deciding which immunotherapeutic agent to choose. According to our findings, Candida antigen can be better tolerated with less adverse events, while DPCP can yield better results in older warts although having more side effects.
Our findings should always be read with some limitations in mind. We were only able to assemble a small sample size, which might affect statistical evaluation and conclusions, and the follow-up period was limited so we still do not have a full perspective of the persistence of the results.
Conclusion
Intralesional Candida antigens and contact DPCP immunotherapy are effective in the treatment of verruca with transient side effects that did not include permanent scarring like other destructive methods. For both modalities, Candida antigen is shown to be the superior treatment option for untreated adjacent warts, a better response for warts with shorter duration, fewer numbers of required treatment sessions and lower risk of side effects. Although immunotherapy has always been discussed as a backup plan for recalcitrant warts, early treatment with Candida antigen injection may yield good results with early intervention.
References
Bruggink SC, Eekhof JA, Egberts PF, van Blijswijk SC, Assendelft WJ, Gussekloo J (2013) Natural course of cutaneous warts among primary schoolchildren: a prospective cohort study. Ann Fam Med 11(5):437–441
Sterling JC, Gibbs S, Haque Hussain SS, Mohd Mustapa MF, Handfield-Jones SE (2014) British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol 171(4):696–712
Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016 Sep-Oct;7(5):364–370. doi: https://doi.org/10.4103/2229-5178.190487. PMID: 27730031; PMCID: PMC5038096
Sinha S, Relhan V, Garg VK. Immunomodulators in warts: Unexplored or ineffective? Indian J Dermatol. 2015 Mar-Apr;60(2):118–29. doi: https://doi.org/10.4103/0019-5154.152502. PMID: 25814698; PMCID: PMC4372902
Majid I, Imran S (2013) Immunotherapy with intralesional Candida albicans antigen in resistant or recurrent warts: a study. Indian J Dermatol 58(5):360–365. https://doi.org/10.4103/0019-5154.117301.PMID:24082180;PMCID:PMC3778775
Brunk D (1999) Injection of Candida antigen works on warts. Skin Allergy News 30:5
Rampen FH, Steijlen PM (1996) Diphencyprone in the management of refractory palmoplantar and periungual warts: an open study. Dermatology 193:236–238
Signore RJ (2002) Candida albicans intralesional injection immunotherapy of warts. Cutis 70(3):185–192
Sil A, Dasgupta S, Chandra S, Datta A, Banerjee A, Das NK (2021) Changes in cytokine profile with immunotherapy in viral warts using purified protein derivative, mumps measles rubella vaccine, and Mycobacterium w Vaccine. Indian J Dermatol 66(1):67–73. https://doi.org/10.4103/ijd.IJD_206_20.PMID:33911296;PMCID:PMC8061473
Park HJ, Choi YW, Kim SH, Shin MS, Lee SW, Oh MK, Choi HY (2013) Change in cytokines in patients with warts after contact immunotherapy with squaric acid dibutylester. Clin Exp Dermatol 38(7):775–781. https://doi.org/10.1111/ced.12075 (Epub 2013 Apr 23 PMID: 23611147)
El-Khalawany M, Shaaban D, Aboeldahab S (2015) Immunotherapy of viral warts: myth and reality. Egypt J Dermatol Venerol 35:1–13
Bacelieri R, Johnson SM (2005) Cutaneous warts: an evidence-based approach to therapy. Am Fam Physician 72:647–652
Johnson SM, Roberson PK, Horn TD (2001) Intralesional injection of mumps or Candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol 137:451–455
Kim KH, Horn TD, Pharis J et al (2010) Phase 1 clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol 146:1431–1433
Nofal A, Salah E, Nofal E, Yosef A (2013) Intralesional antigen immunotherapy for the treatment of warts: current concepts and future prospects. Am J Clin Dermatol 14(4):253–260. https://doi.org/10.1007/s40257-013-0018-8 (PMID: 23813361)
Nassar A et al (2021) "Correlation of serum interleukin 17 and macrophage migration inhibitory factor levels with clinical response to intralesional Candida antigen and their potential use as predictors of clinical outcome in patients with multiple common warts. J Cosmetic Dermatol. https://doi.org/10.1111/jocd.14688
Orecchia G, Douville H, Santagostino L et al (1988) Treatment of multiple relapsing warts with diphenciprone. Dermatologica 177:225–231
Buckley DA, Du Vivier AW (2001) The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol 145:385–405
Pollock B, Highet AS (2002) An interesting response to diphencyprone (DPC) sensitization on facial warts: review of DPC treatment for viral warts. J Dermatolog Treat 13:47–50
Suh DW, Lew BL, Sim WY (2014) Investigations of the efficacy of diphenylcyclopropenone immunotherapy for the treatment of warts. Int J Dermatol 53(12):e567–e571. https://doi.org/10.1111/ijd.12688 (PMID: 25427069)
Khurshid K, Ali U, Pal SS (2009) Role of Candida antigen in treatment of viral warts: a placebo-controlled study. J Pak Assoc Dermatol 19(3):146–150
Phillips RC, Ruhl TS, Pfenninger JL, Garber MR (2000) Treatment of warts with Candida antigen injection. Arch Dermatol 136(10):1274–1275
Miyata K, Go U, Fujita M, Mitsuishi T (2019) Successful Treatment with Topical Diphenylcyclopropenone for Three Cases of Anogenital Warts in Children. Case Rep Dermatol 11(2):123–129. https://doi.org/10.1159/000500295.PMID:31182946;PMCID:PMC6547260
Aghaei S (2006) Treatment of disseminated facial warts through contact immunotherapy with diphenylcyclopropenone (DPCP). Dermatol Online J 12(2):10 (PMID: 16638403)
Van der Steen P, van de Kerkhof P, der Kinderen D, van Vlijmen I, Happle R (1991) Clinical and immunohistochemical responses of plantar warts to topical immunotherapy with diphenylcyclopropenone. J Dermatol 18(6):330–333. https://doi.org/10.1111/j.1346-8138.1991.tb03093.x (PMID: 1939862)
Nofal A, Marei A, Amer A, Amen H (2017) Significance of interferon gamma in the prediction of successful therapy of common warts by intralesional injection of Candida antigen. Int J Dermatol 56(10):1003–1009. https://doi.org/10.1111/ijd.13709 (Epub 2017 Aug 8 PMID: 28791682)
Fathy G, Sharara MA, Khafagy AH (2019) Intralesional vitamin D3 versus Candida antigen immunotherapy in the treatment of multiple recalcitrant plantar warts: A comparative case-control study. Dermatol Ther 32(5):e12997
Choi Y, Kim DH, Jin SY, Lee A-Y, Lee SH (2013) Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment of Periungual Warts. Ann Dermatol 25(4):434–439
Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, Aguilera Peirò P, Baltà Cruz S, Hernández Ruiz ME, Baselga TE (2015) Intralesional Candida Antigen Immunotherapy for the Treatment of Recalcitrant and Multiple Warts in Children. Pediatr Dermatol 32(6):797–801. https://doi.org/10.1111/pde.12667 (Epub 2015 Sep 7 PMID: 26584692)
Hammad NM, Abdelhadi AA, Fawzy MM, Marei A (2020) Complement component 3c and tumor necrosis factor-α systemic assessment after Candida antigen immunotherapy in cutaneous warts. Braz J Microbiol 51(4):1673–1681
Clifton MM, Johnson SM, Roberson PK et al (2003) Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol 20:268–271
Nofal A, Alakad R (2020) Intralesional immunotherapy for the treatment of anogenital warts in pediatric population. J Dermatolog Treat 5:1–5. https://doi.org/10.1080/09546634.2020.1800573
Fields JR, Saikaly SK, Schoch JJ (2020) Intralesional immunotherapy for pediatric warts: A review. Pediatr Dermatol 37(2):265–271. https://doi.org/10.1111/pde.14094 (Epub 2020 Jan 12 PMID: 31930595)
Marei A, Alakad R, Wahid RM (2021) Evaluation of intralesional Candida antigen in diabetic patients with multiple warts. J Cosmet Dermatol 20(4):1248–1253
Suh D-W, Lew B-L, Sim W-Y (2014) Investigations of the efficacy of diphenylcyclopropenone immunotherapy for the treatment of warts. Int J Dermatol 53:567–571
Nofal A, Khedr A, Fathy M (2020) Combined oral isotretinoin and Candida antigen versus either agent alone in the treatment of plane warts. J Dermatolog Treat 22:1–6. https://doi.org/10.1080/09546634.2020.1754325
Nassar A, Mostafa M, Khashaba SA (2020) Photodynamic therapy versus Candida antigen immunotherapy in plane wart treatment: a comparative controlled study. Photodiagnosis Photodyn Ther 32:101973. https://doi.org/10.1016/j.pdpdt.2020.101973 (Epub 2020 Aug 22 PMID: 32841751)
Hodeib AAE, Al-Sharkawy BG, Hegab DS, Talaat RAZ (2021) A comparative study of intralesional injection of Candida albicans antigen, bleomycin and 5-fluorouracil for treatment of plane warts. J Dermatolog Treat 32(6):663–668. https://doi.org/10.1080/09546634.2019.1688236 (Epub 2019 Nov 12 PMID: 31682472)
Marei A, Nofal A, Alakad R, Abdel-Hady A (2020) Combined bivalent human papillomavirus vaccine and Candida antigen versus Candida antigen alone in the treatment of recalcitrant warts. J Cosmet Dermatol 19(3):758–762. https://doi.org/10.1111/jocd.13077 (Epub 2019 Jul 22 PMID: 31328869)
Nofal A, El-Arab RE, Nasr M, Alakad R (2021) Intralesional Measles, Mumps, and Rubella Vaccine Versus Intralesional Candida Antigen in the Treatment of Common and Plantar Warts. J Cutan Med Surg 25(4):377–383. https://doi.org/10.1177/1203475421991130
Fawzy MM, Nofal A, Alakad R (2020) Intralesional antigen immunotherapy for the treatment of plane warts: A comparative study. Dermatol Ther 33(6):e13807. https://doi.org/10.1111/dth.13807
Amer A, Nassar A, Gamal D, Marei A (2021) Effect of varicella zoster vaccine vs Candida antigen injection in treatment of warts. Dermatol Ther 34(1):e14667. https://doi.org/10.1111/dth.14667
Nofal A, Soliman M, Hamdy F, Alakad R (2020) Intralesional Candida antigen versus intralesional tuberculin in the treatment of recalcitrant genital warts: A comparative study. J Am Acad Dermatol 82(6):1512–1514. https://doi.org/10.1016/j.jaad.2019.12.050 (Epub 2020 Jan 7 PMID: 31923442)
Nofal A, Albalat W, Ismail A, Khattab FM (2020) Immunotherapeutic modalities for the treatment of recalcitrant plantar warts: a comparative study. J Dermatolog Treat 9:1–6. https://doi.org/10.1080/09546634.2020.1789540
Attwa E, Elawady R, Salah E (2020) “Cryo-immuno-therapy” is superior to intralesional Candida antigen monotherapy in the treatment of multiple common warts. J Dermatolog Treat 2:1–8. https://doi.org/10.1080/09546634.2020.1720585
Nofal A, Yehia E, Khater E, Bessar H (2020) Alternating intralesional purified protein derivative and Candida antigen versus either agent alone in the treatment of multiple common warts. J Am Acad Dermatol 83(1):208–210. https://doi.org/10.1016/j.jaad.2020.01.054 (Epub 2020 Jan 30 PMID: 32006600)
Mishra A, Uyyala SR (2021) "A novel probabilistic-based deep neural network: toward the selection of wart treatment. Cognit Comput. https://doi.org/10.1007/s12559-021-09882-1
Ghiasi MM, Zendehboudi S (2019) Decision tree-based methodology to select a proper approach for wart treatment. Comput Biol Med 108:400–409
Acknowledgements
Dr. Shahenda Shama had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors has any potential conflict of interest, any relevant financial activities outside the submitted work or any other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, what is written in the submitted work at any time prior to submitting this work. No funding was received for this work. Ethical Committee of the Faculty of Medicine, Helwan university, approved this research in July 2019 with serial number 25/2019. IBR and ethical approval were granted prior to the study and consent was obtained from all participants. Dr. M. Elkomy and Dr. N. Bedair designed the study. Dr. S. Shamma was responsible of recruitment of participants, obtaining their consent, and administrating the therapeutic agents. All authors were responsible for following up the participants. Dr. M. Elkomy and Dr. Nermeen Ibrahim Bedair were responsible for literature review and preparing the draft for this manuscript. All authors approved the manuscript for publishing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
Reviewed and approved by the Faculty of Medicine, Helwan University research ethics committee in July 2019 (serial 25/2019), and the study fulfilled all the ethical aspects and was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
EL-Komy, M.H.M., Shamma, S.G. & Bedair, N.I. The efficacy and safety of intralesional Candida vaccine versus topical diphencyproprobenone in immunotherapy of verruca vulgaris: A randomized comparative study. Arch Dermatol Res 315, 583–591 (2023). https://doi.org/10.1007/s00403-022-02402-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-022-02402-7