Abstract
Bullous pemphigoid (BP) is the most common autoimmune skin disease of blistering character. The underlying pathophysiological mechanism involves an immune attack, usually by IgG class autoantibodies, on the autoantigen BP 180/BPAg2, which is a type XVII collagen (COL17) protein acting as the adhesion molecule between the epidermis and the basement membrane of the dermis. About 40 years ago, following consistent findings of elevated total serum IgE levels in BP patients, it was hypothesized that IgE may be involved in the pathophysiology of BP. Our objective was to determine whether there is strong evidence for an association between IgE class autoantibodies and the clinical severity or phenotype of BP. Three databases were searched for relevant studies and appropriate exclusion and inclusion criteria were applied. Data was extracted and assessed in relation to the study questions concerning the clinical significance of IgE autoantibodies in BP. Nine studies found that anti-BP180 autoantibodies of IgE class are associated with increased severity of BP, whereas two studies did not find such an association. The number of studies which found an association between higher IgE autoantibody levels and the erythematous urticarial phenotype of BP (5) was equal in number to the studies which found no such association (5). In conclusion, higher serum IgE autoantibody levels are associated with more severe clinical manifestations of BP. There is insufficient evidence to support higher IgE autoantibody levels being associated with specific clinical phenotypes of BP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
IgE has traditionally been linked to allergic Type 1 hypersensitivity reactions. However, just over four decades ago, antinuclear autoantibodies of IgE class were detected in RA and SLE patients and it was hypothesized that IgE may have a pathophysiological role in autoimmunity [45]. This association between IgE and autoimmunity was a revolutionary concept in the field of hypersensitivity.
A pathophysiological role of IgE is observed in several autoimmune diseases ranging from systemic ones, like SLE [16], to tissue-specific ones, like Graves’ disease [50]. Furthermore, IgE autoantibodies also seem to be pathogenic in certain allergic conditions, like atopic dermatitis (AD). It is believed that IgE autoantibodies may attack keratinocytes in AD [1], an observation which raises questions as to whether AD is primarily atopic or autoimmune in nature, or a combination of both.
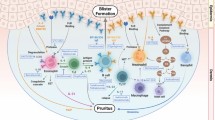
Pemphigoid is a group of autoimmune disorders in which autoantibodies attack the structural proteins comprising the junction of the epidermal and dermal layers of the skin [52]. Pemphigoid disorders manifest themselves clinically through severe blistering of the skin and superficial layers of the mucosa [52]. BP is the most common pemphigoid disorder, accounting for ~ 80% of all cases [49], and is the most common skin autoimmune disease of blistering character. It affects males and females equally, and it is considered mainly a disorder of old age, with the mean age of disease onset being 80. In the UK, it is estimated that there are 43 cases of BP per 1 million of the population [58], with a doubling of reported cases across Europe in the past decade [52]. BP causes relapses of inflammation in the sub-epidermal skin layers, resulting in local or widespread blisters, rash and pruritus. The underlying pathophysiological mechanisms involve an immune attack on the autoantigen BP 180/BPAg2, which is a type XVII collagen (COL17) protein acting as the adhesion molecule between the epidermis and the basement membrane of the dermis. What distinguishes BP from other autoimmune blistering skin diseases is the presence of accumulated IgG on the superficial surface (rather than the dermal surface) of the epidermis in the blistered areas of the skin. Treatment is heavily dependent on the use of topical and systemic corticosteroids.
Studies on BP patients show elevated IgE class autoantibodies with a pathophysiological role. Evidence lies within the detection of IgE autoantibodies targeting the BP180 adhesion molecule and leading to the manifested symptoms [18]. In addition, omalizumab, a humanized monoclonal anti-IgE antibody that was originally designed to control the symptoms of persisting severe asthma following corticosteroid and β2 agonist treatment [9], has proven to be effective in treating the symptoms of BP in human subjects [20, 21].
With this information in hand, we decided to carry out a systematic literature review to determine, through analysis of the studies available, whether there is validity in the findings of IgE autoantibodies in BP, and if so, whether the presence of elevated IgE autoantibody levels affects the severity of the phenotype and/or the course of the disease.
Methods
MEDLINE was accessed through PubMed, EMBASE through OVID and WEB OF SCIENCE through the Wiley Online Library. Each database was searched with the term ‘(ige or immunoglobulin e) and (bullous pemphigoid or bp)’ on 15 October 2016. This yielded a total of 1084 records as follows: MEDLINE, 496; EMBASE, 31; WEB OF SCIENCE, 557. At this initial stage, for comparison between disease, searches were also performed for IgE in chronic idiopathic urticaria (656 records) and IgE autoantibodies in atopic dermatitis (281 records), giving a total of 2021 records. Figure 1 shows the PRISMA 2009 flow diagram used to illustrate the study selection process for the systematic review. This shows the process of searching for records, removing duplicates, screening titles and abstracts, assessing full texts for eligibility and selecting which studies would be used in the analysis. This yielded 33 studies of which 19 were used to document the chronological development of the occurrence of IgE autoantibodies in BP, and 16 provided information to assess the contribution of IgE autoantibodies to the severity and/or clinical phenotype of BP (2 studies provided information relevant to both parts of the review). As illustrated in Fig. 2, of all papers published on the topic in the past 42 years, 50% were published in the last 6 years (2010–2016). This indicates a rapid expansion of interest and investigation in the topic in recent years.
Results and discussion
Chronological development in the field of the relationship of IgE and BP
IgE and the pathophysiology of BP
The first findings that led to the concept that IgE may be linked to BP were published in 1974. Following this, in the 1970s and 1980s, a variety of studies were conducted to address this hypothesis. During these two decades, blood sampling and testing, immunofluorescence on lesioned skin biopsies and chromatography using dermal infiltrate were the main laboratory techniques employed in this research. As is clearly illustrated below, the role of IgE in BP during this time was only demonstrated in a non-specific fashion: for example, through findings of IgE deposition in skin basement membrane (BM), elevated serum IgE levels and peripheral blood eosinophilia. Inevitably, it was unclear whether the apparent involvement of IgE in BP was of a pathogenic nature, or whether it was simply an epiphenomenon of the disease itself.
In 1974, elevated IgE levels were reported in 70% of patients with bullous pemphigoid [3]. In the same year, a study using direct and indirect immunofluorescence detected moderate staining of IgE on the skin BM of four (out of 16) BP patients, one of whom also had greatly elevated serum IgE [47]. Two years later, a study of tissue eosinophilia in BP lesions of four patients showed eosinophil chemotactic activity in the infiltrate [5]; furthermore, the serum of half of the subjects tested positive for IgE autoantibodies to skin BM. In 1980, a similar study reported that 11 of 25 BP patients had elevated serum IgE levels, and 15 of them tested positive for serum IgE anti-BM autoantibodies by indirect immunofluorescence [44]. A study in 1983 on 28 BP patients found 50% to have peripheral blood eosinophilia; the authors proposed that BP should be considered in the differential diagnosis of skin bullous conditions with peripheral blood eosinophilia [8].
In the early 1990s, the application of newer techniques, such as ELISA, enabled better definition of the relationship between IgE and BP, as it was then possible to identify specific receptors for IgE that were possibly involved in the pathophysiology. By the mid-1990s, it was established that BP involved impaired B cell function, and the soluble form of CD23 (sCD23), a low affinity IgE receptor (FcεRII) on haematopoietic cells, was known to be involved in B lymphocyte growth and differentiation. As a result, the role of CD23 in the pathophysiology of BP was investigated and elevated levels of sCD23 were detected in the sera of BP patients [24]. Furthermore, there was significant correlation between sCD23 and serum IgE levels in the BP patients; this correlation was not apparent in the sera of non-BP control subjects. The authors concluded that sCD23 was an important index for monitoring the disease in relation to IgE abnormalities and impaired B lymphocyte function. A year later, the understanding of the involvement of sCD23 in BP was further advanced when it was reported that levels of sCD23 in the dermal bullae infiltrates of ten BP patients were significantly higher than in the infiltrates of suction bullae formed in ten non-BP control subjects [51]. Again, there was correlation between the serum levels of sCD23 and IgE levels in all ten BP patients.
Some pivotal studies in the development of the field showed specific autoantigens in the hemidesmosomal proteins of the skin to be the targets of autoreactive IgE antibodies. As discussed below, BP180 and BP230 are major autoantigens targeted by IgE autoantibodies in BP. BP180 (BPAg2), a transmembrane protein, was the first autoantigen characterized in BP and was found to have collagenous repeats in its ectodomain capable of forming collagen-like helices [27]; indeed, BP180 was later renamed Collagen XVII. BP230 (BPAg1) was first identified using sera from BP patients and was found to be located on the intracellular domain of the hemidesmosome where it functions as a plakin family protein, associating keratin filaments to the hemidesmosome [53]. As a consequence of identifying these two proteins as targets of IgE autoantibodies, by the mid-1990s, the role of IgE in BP was more widely accepted as being pathogenic rather than being an epiphenomenon.
A study in 1996 using a radioimmunoassay reported 12 out of 19 BP patients to have IgE autoantibodies against the BP55 antigen [15]. Two years later, a study examined the antigenic specificity of IgE autoantibodies in 39 BP serum samples using ELISA, immunoblotting and immunofluorescence microscopy: 18 of the samples contained IgE autoantibodies against either the BM or a 230 kDa epidermal antigen (BP230) [25]. The epitopes recognized by the autoantibodies mapped primarily to the C-terminal end of the protein and there was strong correlation between circulating IgE autoantibodies and serum IgE levels. However, this study found no sera containing anti-BP180 IgE autoantibodies. In 2000, immunoblotting and ELISA were used to show that IgE and IgG4 isotypes preferentially react with two epitopes within the ectodomain (NC16A) of BP180 [18]. Another study used double-labelling immunofluorescence to identify a potential effector function of IgE autoantibodies associated with their reactivity to BP180 auto-antigen: of the 30 BP patients studied, 70% of untreated patients had elevated total IgE levels and 86% had detectable anti-BP180 IgE in their serum [17]. IgE-coated mast cells were detected in perilesional skin, which were also coated with BP180 peptides. BP180-stimulated histamine release was notably higher in basophils obtained from BP patients who received no treatment in contrast to patients who either received treatment for BP or the healthy control subjects. The attack on BP180 by autoreactive IgE continued to be the main focus of the investigations for the rest of the decade. Fine mapping of the antigenic sites of NC16A ectodomain of BP180 showed similar reactivity patterns for IgE and IgG, with subregion-2 being the major site recognized by both [23]. However, antigen-specific histamine release by basophils occurred only in the presence of IgE autoantibodies specific for NC16A. In 2009, a study using immunoblotting found that 16 of 18 serum samples contained autoreactive IgE specific for an epitope of the intracellular domain (ICD) of BP180, suggesting that the ectodomain NC16A is not the only target region of IgE on BP180 [19]. The target sites on the ICD of BP180 play an important role in the incorporation of proteins into hemidesmosomal structures. The authors suggested that this could be a possible pathogenic mechanism of IgE in BP, since tampering with the interaction of BP180 with other hemidesmosomal constituents could lead to the dermal–epidermal separation observed in the affected skin of BP patients.
An interesting recent observation concerning the pathophysiology of IgE in BP indicates that IgE may have FcR-independent effects: IgE was found to stimulate FcR-independent release of IL-6 and IL-8 by keratinocytes in vitro [41]. When the experiment was repeated using an organ (skin) culture, IL-6 and IL-8 were similarly released. Cytokine release was also accompanied by a decrease in the number of hemidesmosomal proteins located in the BM zone.
IgE and the diagnosis of BP
A highly sensitive method for detecting anti-BP180 NC16A IgE autoantibodies in sera of BP patients was reported in 2009 [39]. The assay detected IgE autoantibodies in 77% of sera tested, a frequency significantly higher than previously reported and equivalent to that of anti-BP180 NC16A IgG autoantibodies. The results of the study ranked the ELISA method as the most sensitive, when compared with other techniques such as immunoblotting and IIF, and that testing for both IgG and IgE anti-BP180 NC16A may be a more efficient indicator of disease severity and predictor of treatment effectiveness. Nevertheless, the authors stressed that NC16A is not the only target of anti-BP180 autoantibodies, and that BP180 is not the only autoantigenic target in BP. Thus, an NC16A-specific ELISA alone may underestimate the actual reactivity of autoantibodies with BP180 and other autoantigens in BP.
More recently, Pomponi et al. evaluated the efficiency of the ISAC® microarray system in detecting IgE and IgG antibodies in BP patients [46]. The experimental version of ISAC® that was used included BP180 NC16A and was able to replicate the results of ELISA. These improvements in the diagnostic techniques for the detection of autoantibodies involved in BP are of great importance in reducing discrepancies that arise in the literature due to the heterogeneity in the sensitivity of various laboratory assays.
IgE and the treatment of BP
The hypothesis of IgE having a pathogenic role in BP has been further established through the successful treatment of BP patients with omalizumab, a humanized monoclonal anti-IgE antibody which binds to IgE [60]. In 2009, Fairley et al. reported the use of omalizumab to treat a BP patient who was unresponsive to steroid treatment [21]. The team justified the decision based on the study of Dimson et al. [17] which reported that 70% of untreated BP patients presented with elevated total serum IgE levels and 86% presented with anti-BP180 NC16A IgE autoantibodies. Clinical improvement was observed within 16 weeks of commencing omalizumab treatment. By week 1 of treatment, clinical improvements included a decrease in pruritus and in bullous count by 44%. By week 16, there was a 45% decline in the skin involved in urticarial lesions and a drop in peripheral blood eosinophil count from 3427 to 887/mm3 (normal ≤ 475/mm3). Four months after discontinuation of treatment, the patient reported pruritus and new bullae formation, but re-initiation of omalizumb resulted in resolution of bullae and subsidence of pruritus. This was the first treatment targeting IgE in a BP patient and it was also the first in vivo demonstration of the pathogenicity of IgE class autoantibodies in BP. Similarly, in 2012, Dufour et al. [20] reported the treatment with omalizumab of a 5-month-old boy suffering from infant BP (IBP), who was unresponsive to steroids. Omalizumab treatment resulted in a decline in the number of bullae and urticarial lesions and overall disease control was achieved by day 25 of treatment. Omalizumab was continued for the next 7 months and no clinical relapse occurred during this period. This observation supported the efficacy of omalizumab in treating IBP. Following these reports of single patient cases, a study was undertaken in which six patients were treated with omalizumab and monitored for up to 42 months [60]. Five of them benefited therapeutically: there was inhibition of new bullae formation, subsidence of pruritus, a decline in eosinophil count and a decreased need for immunosuppressant medication. Despite this being an uncontrolled study, the authors felt that the results were sufficiently clear-cut to conclude that omalizumab can neutralize IgE reactivity in patients with BP and control the disease.
IgE in relation to disease activity and clinical phenotype of BP
As the role of IgE in the pathophysiology of BP became better understood as a pathogenic one, hypotheses about how its presence in BP may affect the disease severity/activity and/or clinical manifestations (i.e. phenotype) were put forward. Of the earliest papers to explore a possible correlation between IgE and BP activity was that of Asbrink and Hovmark [4], which suggested that total serum IgE was a better measure of disease activity in contrast to IIF. Since then, several studies conducted in the field have addressed how total serum IgE or, more commonly, the levels of IgE autoantibodies in the serum, can potentially affect the disease severity/activity and/or the phenotype of BP. This review has assessed the outcomes of 16 primary studies (details summarized in Table 1a, b) conducted between 2000 and 2016, and one Letter to the Editor, which address this issue.
IgE and the disease severity/activity of BP
Disease severity of BP has been defined in a variety of ways over the years. The severity of BP used to be assessed by counting the number of bullae on the skin of the patient. Joly et al. and Bernard et al. [6, 33] reported the use of this method: the larger the number of bullae, the more points were allocated for disease severity. Tsuji-Abe et al. and Roujeau et al. [48, 55] reported the use of a similar method, but instead of counting individual bullae, it was the percentage of skin affected by lesions that was taken into consideration. Ishiura et al. [31], Iwata et al. [32] and Messingham et al. [39] also reported the use of this method and the development of scales (unofficial, self-defined scoring scales) for quantifying disease severity. In 2012, the bullous pemphigoid disease area index (BPDAI) was introduced. This is an objective scale which continues to be used for assessing the % of skin affected by BP-related lesions (both bullous and erythematous) and degree of pruritus [34]. The scale ranges from 0 to 120 and the total BPDAI score can be calculated by adding together the scores for bullae, erythematous lesions and pruritus [57].
As shown in Table 2, nine studies [7, 11, 18, 26, 32, 34, 39, 40, 57] found that anti-BP180 autoantibodies of IgE class are associated with increased severity of BP, in contrast to two studies [38, 42] which did not find such an association. The two studies which reported no association did not seem to have any major differences in conduct from the rest of the studies which did report an association. No studies reported an association between anti-BP230 IgE autoantibodies and disease severity, while four studies [26, 31, 32, 42] stated that anti-BP230 IgE autoantibody serum levels or deposition in the skin BM showed no correlation with disease severity.
Since it has been demonstrated that total serum IgE levels correlate directly with the levels of IgE specific for BP180 and BP230 antigens [35] and that there is a good positive correlation between circulating IgE autoantibodies and total serum IgE [25], some studies have sought to determine whether disease severity correlates with total serum IgE levels: three studies reported a positive correlation between total serum IgE levels [11, 34, 35] and disease severity and one study [14] reported no correlation.
Overall, the high proportion of studies reporting an association between anti-BP180 IgE autoantibodies and severity of BP compared to the studies reporting no such association (9:2) favours a correlation between the presence of IgE autoantibodies and increased disease severity.
IgE and the clinical presentation of BP
Bullous pemphigoid does not manifest as a single phenotype. The typical bullous phenotype involves local or widespread blisters and eruptions; however, these can be preceded by lesions of an urticarial nature on erythematous skin. One of the less common forms of BP is pemphigoid nodularis (nodular phenotype) which is characterized by highly pruritic lesions, often formed on nodular skin [12].
Table 3 shows the numbers of studies that have reported correlation (or lack of correlation) of various IgE parameters to the different phenotypes of BP [11, 14, 26, 42, 57, 59]. Overall, these studies indicate that IgE autoantibodies are not specifically associated with the blisters and eruptions seen in the typical presentation of BP. The urticarial presentation of the condition has been the most investigated phenotype of BP with respect to parameters of IgE expression. However, the number of reports that IgE (total or autoantibody) levels are associated with urticarial lesions is equal to the number that found no such associations. There is thus insufficient evidence at present to conclude from studies in patients that IgE autoantibodies are associated with specific clinical phenotypes of BP.
Amber [2] suggested that two possible problems in establishing an association between IgE autoantibodies and the urticarial stage of BP could be variable sensitivity in detecting IgE autoantibodies in BP patients and insufficient discrimination of distinct clinical phenotypes within the spectrum of the disease (see also reply by Zuo [63]). Two other lines of investigation that indirectly provide evidence relevant to this issue are studies of BP in murine models, and studies of IgE autoantibodies in other skin diseases:
Firstly, whereas IgG-mediated murine models lack urticarial erythema and eosinophil infiltration, these features occur in human skin engrafted on to SCID mice inoculated with monoclonal IgE specific to the BP180 ectodomain [62]. A similar model has been developed using human skin-engrafted nude mice inoculated with IgE from BP patients [22]. Direct immunization of mice with fragments of BP180 also induces skin lesions associated with raised IgE and infiltration of eosinophils [28].
Secondly, autoantibodies of IgE class have been reported in patients with other inflammatory skin diseases. IgE autoantibodies specific for double-stranded DNA [10] and for thyroid autoantigens [10, 13] have been described in some patients with chronic idiopathic urticaria; these autoantibodies may contribute to disease symptoms by activating mast cells and basophils [37]. IgE autoantibodies specific for a variety of autoantigens have been reported in some atopic dermatitis patients [29, 30, 43, 54, 56, 61] and appear to correlate with disease severity and chronicity [36, 54].
Limitations of this systematic review
As shown in Table 1, the studies used in this review were subject to several limitations. Most were conducted in a single-centre fashion, and only a few countries (notably, the USA, Japan, China and Germany) have been conducting the majority of research in this area. As a result, it is not known whether the conclusions of these studies are generalizable to all ethnicities. In addition, most of the studies used sample sizes that are small—this problem may arise because of the relative rarity of BP. In general, little information has been given about the subjects involved, other than sex and age; for example, about their overall health and any comorbidities they have that could constitute confounding factors, or about the methods whereby they were recruited. Lack of such information could be a warning for selection, exclusion and sampling bias. Another potential problem is the ambiguity in the full spectrum of signs, symptoms and phenotypes of BP, leading to a vague consensus on the manifestations of the disease. This poses a risk of classification bias when selecting patients for a study. Moreover, procedural errors could lead to bias as well. For instance, several studies have reported an uncertain degree of sensitivity of the methods used in determining IgE autoantibody concentrations, and blinding of the scientists conducting the experiments is not often mentioned, raising possible detection bias. The diversity in the scales used to assess the disease severity of BP contributes further to procedural inaccuracies. The lack of healthy controls and disease comparisons in numerous studies poses a risk of yielding results of questionable reliability. Furthermore, most studies included in this review are retrospective studies, which pose an increased risk of recall bias and the influence of confounding factors compared to prospective studies. As with all systematic reviews, there is inevitably a risk of publication bias as the conclusions of the review are based on published data, which can be a problem since positive outcomes are three times more likely to be published than are negative outcomes. No grey literature was used in this review and some data were unavailable for us to use. Furthermore, there is a high degree of heterogeneity in the studies used, particularly with respect to the sample sizes and characteristics, the criteria for diagnosing BP, the type of variables investigated and the methods used for measuring the variables, the use of control/comparison groups and the actual outcomes.
Conclusion
This review’s results support the conclusion that the higher the serum IgE autoantibody levels are, the more severe the manifestation of BP will be; however, they do not support the possibility that higher IgE autoantibody levels promote a more urticarial presentation of BP. It is fair to say that there is ambiguity as to whether IgE can be held accountable for the full spectrum of BP manifestations, or whether its effects are more relevant to the initial stages of the disease. Further research is needed in the future to establish more solid conclusions. There is a need for more multi-centre prospective cohort studies which would help to eliminate uncertainties in the field, particularly with respect to a possible association between the occurrence of IgE class autoantibodies and the clinical phenotype of BP.
References
Altrichter S, Kriehuber E, Moser J, Valenta R, Kopp T, Stingl G (2008) Serum IgE autoantibodies target keratinocytes in patients with atopic dermatitis. J Invest Dermatol 128:2232–2239
Amber KT (2016) Is anti-BP180 IgE associated with clinical phenotype? A reply to ‘Levels of anti-BP180 NC16A IgE do not correlate with severity of disease in the early stages of bullous pemphigoid’. Arch Dermatol Res 308:65–66
Arbesman CE, Wypych JI, Reisman RE, Beutner EH (1974) IgE levels in sera of patients with pemphigus or bullous pemphigoid. Arch Dermatol 110:378–381
Asbrink E, Hovmark A (1984) Serum IgE levels in patients with bullous pemphigoid and its correlation to the activity of the disease and anti-basement membrane zone antibodies. Acta Derm Venereol 64:243–246
Baba T, Sonozaki H, Seki K, Uchiyama M, Ikesawa Y, Toriisu M (1976) An eosinophil chemotactic factor present in blister fluids of bullous pemphigoid patients. J Immunol 116:112–116
Bernard P, Venot J, Constant F, Bonnetblanc JM (1987) Blood eosinophilia as a severity marker for bullous pemphigoid. J Am Acad Dermatol 16:879–881
Bing L, Xiping Z, Li L, Jun P, Yi-Xia W, Min Y, Qing L, Qiu-Ning S, Hong-Zhong J, Ya-Gang Z (2015) Levels of anti-BP180 NC16A IgE do not correlate with severity of disease in the early stages of bullous pemphigoid. Arch Dermatol Res 307:849–854
Bushkell LL, Jordon RE (1983) Bullous pemphigoid: a cause of peripheral blood eosinophilia. J Am Acad Dermatol 8:648–651
Canonica GW, Senna G, Mitchell PD, O’Byrne PM, Passalacqua G, Varricchi G (2016) Therapeutic interventions in severe asthma. World Allergy Organ J 9:40
Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M (2015) The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol 135:337–342
Cho YT, Liao SL, Wang LF, Chu CY (2016) High serum anti-BP180 IgE levels correlate to prominent urticarial lesions in patients with bullous pemphigoid. J Dermatol Sci 83:78–80
Cliff S, Holden CA (1997) Pemphigoid nodularis: a report of three cases and review of the literature. Br J Dermatol 136:398–401
Concha LB, Chang CC, Szema AM, Dattwyler RJ, Carlson HE (2004) IgE antithyroid antibodies in patients with Hashimoto’s disease and chronic urticaria. Allergy Asthma Proc 25:293–296
Cozzani E, Parodi A, Rebora A, Delmonte S, Barile M, Nigro A, Priano L, Troiano G, Patri PL, Gruppo Liguredi Studi in D (2001) Bullous pemphigoid in Liguria: a 2-year survey. J Eur Acad Dermatol Venereol 15:317–319
Delaporte E, Dubost-Brama A, Ghohestani R, Nicolas JF, Neyrinck JL, Bergoend H, Janin A, Capron M (1996) IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol 157:3642–3647
Dema B, Pellefigues C, Hasni S, Gault N, Jiang C, Ricks TK, Bonelli MM, Scheffel J, Sacré K, Jablonski M, Gobert D, Papo T, Daugas E, Illei G, Charles N, Rivera J (2014) Autoreactive IgE Is prevalent in systemic lupus erythematosus and is associated with increased disease activity and nephritis. PLoS ONE 9:e90424
Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, Fairley JA (2003) Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol 120:784–788
Dopp R, Schmidt E, Chimanovitch I, Leverkus M, Brocker EB, Zillikens D (2000) IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol 42:577–583
Dresow SK, Sitaru C, Recke A, Oostingh GJ, Zillikens D, Gibbs BF (2009) IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol 160:429–432
Dufour C, Souillet AL, Chaneliere C, Jouen F, Bodemer C, Jullien D, Cambazard F, Joly P, Reix P (2012) Successful management of severe infant bullous pemphigoid with omalizumab. Br J Dermatol 166:1140–1142
Fairley JA, Baum CL, Brandt DS, Messingham KA (2009) Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol 123:704–705
Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ (2007) A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol 127:2605–2611
Fairley JA, Fu CL, Giudice GJ (2005) Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. J Invest Dermatol 125:467–472
Furukawa F, Kumagai S, Sakamoto Y, Takigawa M, Imamura S (1994) Elevated serum levels of IgE-binding factor/soluble CD23 in bullous pemphigoid. J Dermatol Sci 7:150–154
Ghohestani RF, Cozzani E, Delaporte E, Nicolas JF, Parodi A, Claudy A (1998) IgE antibodies in sera from patients with bullous pemphigoid are autoantibodies preferentially directed against the 230-kDa epidermal antigen (BP230). J Clin Immunol 18:202–209
Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D, Hachiya T, Kuroda K, Hashiguchi M, Kawakami T, Ishii N (2016) Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol 177:141–151
Hirako Y, Usukura J, Nishizawa Y, Owaribe K (1996) Demonstration of the molecular shape of BP180, a 180-kDa bullous pemphigoid antigen and its potential for trimer formation. J Biol Chem 271:13739–13745
Hirose M, Recke A, Beckmann T, Shimizu A, Ishiko A, Bieber K, Westermann J, Zillikens D, Schmidt E, Ludwig RJ (2011) Repetitive immunization breaks tolerance to type XVII collagen and leads to bullous pemphigoid in mice. J Immunol 187:1176–1183
Hradetzky S, Roesner LM, Balaji H, Heratizadeh A, Mittermann I, Valenta R, Werfel T (2014) Cytokine effects induced by the human autoallergen alpha-NAC. J Invest Dermatol 134:1570–1578
Hradetzky S, Werfel T, Rosner LM (2015) Autoallergy in atopic dermatitis. Allergo J Int 24:16–22
Ishiura N, Fujimoto M, Watanabe R, Nakashima H, Kuwano Y, Yazawa N, Echigo T, Okochi H, Tamaki K (2008) Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci 49:153–161
Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, Muroi E, Ogawa F, Takenaka M, Sato S (2008) Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol 144:41–48
Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, Vaillant L, D’Incan M, Plantin P, Bedane C, Young P, Bernard P, Bullous Diseases French Study G (2002) A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med 346:321–327
Kalowska M, Ciepiela O, Kowalewski C, Demkow U, Schwartz RA, Wozniak K (2016) Enzyme-linked immunoassay index for anti-NC16a IgG and IgE auto-antibodies correlates with severity and activity of bullous pemphigoid. Acta Derm Venereol 96:191–196
Kelly LA, Holubkov R, Campbell MA, Herlevi KS, Taylor T, Hull C, Gleich GJ, Zone JJ, Leiferman KM (2007) Serum IgE concentrations in bullous pemphigoid correlate with disease activity. J Allergy Clin Immunol 119:S204
Kinaciyan T, Natter S, Kraft D, Stingl G, Valenta R (2002) IgE autoantibodies monitored in a patient with atopic dermatitis under cyclosporin A treatment reflect tissue damage. J Allergy Clin Immunol 109:717–719
Kolkhir P, Pogorelov D, Olisova O, Maurer M (2016) Comorbidity and pathogenic links of chronic spontaneous urticaria and systemic lupus erythematosus—a systematic review. Clin Exp Allergy 46:275–287
Ma L, Wang M, Wang X, Chen X, Zhu X (2015) Circulating IgE anti-BP180 autoantibody and its correlation to clinical and laboratorial aspects in bullous pemphigoid patients. J Dermatol Sci 78:76–77
Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA (2009) A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods 346:18–25
Messingham KN, Holahan HM, Frydman AS, Fullenkamp C, Srikantha R, Fairley JA (2014) Human eosinophils express the high affinity IgE receptor, FcepsilonRI, in bullous pemphigoid. PLoS ONE 9:e107725
Messingham KN, Srikantha R, DeGueme AM, Fairley JA (2011) FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol 187:553–560
Moriuchi R, Nishie W, Ujiie H, Natsuga K, Shimizu H (2015) In vivo analysis of IgE autoantibodies in bullous pemphigoid: a study of 100 cases. J Dermatol Sci 78:21–25
Muro Y (2001) Autoantibodies in atopic dermatitis. J Dermatol Sci 25:171–178
Nieboer C, van Leeuwen HJ (1980) IgE in the serum and on mast cells in bullous pemphigoid. Arch Dermatol 116:555–556
Permin H, Wiik A (1978) The prevalence of IgE antinuclear antibodies in rheumatoid arthritis and systemic lupus erythematosus. Acta Pathol Microbiol Scand C 86C:245–249
Pomponi D, Di Zenzo G, Zennaro D, Calabresi V, Eming R, Zuzzi S, Bernardi ML, Scala E, Mari A (2013) Detection of IgG and IgE reactivity to BP180 using the ISAC(R) microarray system. Br J Dermatol 168:1205–1214
Provost TT, Tomasi TB Jr (1974) Immunopathology of bullous pemphigoid. Basement membrane deposition of IgE, alternate pathway components and fibrin. Clin Exp Immunol 18:193–200
Roujeau JC, Lok C, Bastuji-Garin S, Mhalla S, Enginger V, Bernard P (1998) High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol 134:465–469
Sanjuan MA, Sagar D, Kolbeck R (2016) Role of IgE in autoimmunity. J Allergy Clin Immunol 137:1651–1661
Sato A, Takemura Y, Yamada T, Ohtsuka H, Sakai H, Miyahara Y, Aizawa T, Terao A, Onuma S, Junen K, Kanamori A, Nakamura Y, Tejima E, Ito Y, Kamijo K (1999) A possible role of immunoglobulin E in patients with hyperthyroid Graves’ disease. J Clin Endocrinol Metab 84:3602–3605
Schmidt E, Brocker EB, Zillikens D (1995) High levels of soluble CD23 in blister fluid of patients with bullous pemphigoid. Arch Dermatol 131:966–967
Schmidt E, Zillikens D (2013) Pemphigoid diseases. Lancet 381:320–332
Stanley JR (1993) Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol 53:291–325
Tang TS, Bieber T, Williams HC (2012) Does “autoreactivity” play a role in atopic dermatitis? J Allergy Clin Immunol 129(1209–1215):e2
Tsuji-Abe Y, Akiyama M, Yamanaka Y, Kikuchi T, Sato-Matsumura KC, Shimizu H (2005) Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci 37:145–149
Valenta R, Natter S, Seiberler S, Wichlas S, Maurer D, Hess M, Pavelka M, Grote M, Ferreira F, Szepfalusi Z, Valent P, Stingl G (1998) Molecular characterization of an autoallergen, Hom s 1, identified by serum IgE from atopic dermatitis patients. J Invest Dermatol 111:1178–1183
van Beek N, Luttmann N, Huebner F, Recke A, Karl I, Schulze FS, Zillikens D, Schmidt E (2017) Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol 153:30–38
Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirtschig G (2012) British association of dermatologists’ guidelines for the management of bullous pemphigoid 2012. Br J Dermatol 167:1200–1214
Yayli S, Pelivani N, Beltraminelli H, Wirthmuller U, Beleznay Z, Horn M, Borradori L (2011) Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: a useful clue for diagnosis. Br J Dermatol 165:1133–1137
Yu KK, Crew AB, Messingham KA, Fairley JA, Woodley DT (2014) Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol 71:468–474
Zeller S, Rhyner C, Meyer N, Schmid-Grendelmeier P, Akdis CA, Crameri R (2009) Exploring the repertoire of IgE-binding self-antigens associated with atopic eczema. J Allergy Clin Immunol 124:278–285
Zone JJ, Taylor T, Hull C, Schmidt L, Meyer L (2007) IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol 127:1167–1174
Zuo Y (2016) Is anti-BP180 IgE associated with clinical phenotype? Reply to the letter to the editor. Arch Dermatol Res 308:67–68
Related articles recently published in Archives of Dermatological Research (selected by the journal’s editorial staff)
Kalinska-Bienias A, Lukowska-Smorawska K, Jagielski P, Kowalewski C, Wozniak K (2017) Mortality in bullous pemphigoid and prognostic factors in 1st and 3rd year of follow-up in specialized centre in Poland. Arch Dermatol Res. doi:10.1007/s00403-017-1772-x
Keller JJ, Kittridge AL, Debanne SM, Korman NJ (2016) Evaluation of ELISA testing for BP180 and BP230 as a diagnostic modality for bullous pemphigoid: a clinical experience. Arch Dermatol Res 308:269–272
Liu YD, Wang YH, Ye YC, Zhao WL, Li L (2017) Prognostic factors for mortality in patients with bullous pemphigoid: a meta-analysis. Arch Dermatol Res 309:335–347
Mersmann M, Dworschak J, Ebermann K, Komorowski L, Schlumberger W, Stocker W, Zillikens D, Probst C, Schmidt E (2016) Immunoadsorber for specific apheresis of autoantibodies in the treatment of bullous pemphigoid. Arch Dermatol Res 308:31–38
Thorslund K, Seifert O, Nilzen K, Gronhagen C (2017) Incidence of bullous pemphigoid in Sweden 2005–2012: a nationwide population-based cohort study of 3761 patients. Arch Dermatol Res. doi:10.1007/s00403-017-1778-4
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saniklidou, A.H., Tighe, P.J., Fairclough, L.C. et al. IgE autoantibodies and their association with the disease activity and phenotype in bullous pemphigoid: a systematic review. Arch Dermatol Res 310, 11–28 (2018). https://doi.org/10.1007/s00403-017-1789-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-017-1789-1