Abstract
Leishmania (Viannia) shawi causes cutaneous lesions in humans. Parasite antigens conferring significant protection against American tegumentar leishmaniosis (ATL) might be important for the development of effective vaccine. Therefore, this work evaluates the protective effect of antigenic fractions released by L. shawi. Antigens released by promastigotes to culture medium were concentrated and isolated by SDS-PAGE. The three main fractions LsPass1 (>75 kDa), LsPass2 (75–50 kDa) and LsPass3 (<50 kDa) were electro-eluted according with their molecular mass. Immunized BALB/c mice were challenged with L. shawi promastigotes and the course of infection monitored during 5 weeks. LsPass1-challenged mice showed no protection, however, a strong degree of protection associated to smaller lesions and high expression of IFN-γ and TNF-α by CD4+ T, CD8+ T and double negative CD4CD8 cells was achieved in LsPass3-challenged mice. Furthermore, LsPass2-challenged mice showed an intermediated degree of protection associated to high levels of IFN-γ, IL-4 and IL-10 mRNA. In spite of increased expression of IFN-γ and TNF-α, high amounts of IL-4 and IL-10 mRNA were also detected in LsPass3-challenged mice indicating a possible contribution of these cytokines for the persistence of a residual number of parasites that may be important in inducing long-lasting immunity. Therefore, LsPass3 seems to be an interesting alternative that should be considered in the development of an effective vaccine against ATL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniosis are diseases caused by the parasite of the genus Leishmania. This parasite infects a broad range of mammalian species, generating tegumentar or visceral leishmaniosis. Although over 27 species of Leishmania have been identified worldwide, there are some species recently identified in New World. Studies able to understand the dynamics of epidemiology and the immune-pathogenesis of these newly identified species are scarce [11, 13, 14, 37, 38].
Leishmania (Viannia) shawi was described by Lainson and collaborators in 1989 [13] in non-human primates from Pará State, Brazil, and recognized as pathogenic to humans 2 years after [37]. Furthermore, Brito and collaborators [2] described five new L. shawi isolates obtained from clinical cases identified in Pernambuco State suggesting that this parasite may have a non-negligible epidemiological importance in Brazil. Recently, our group has characterized the murine model of L. shawi infection [27], evaluating cellular and humoral immune responses in infected BALB/c mice to better clarify the main immunological alterations induced by this parasite, and consequently to establish a standardized susceptible animal model for further studies [28].
Murine models of tegumentar leishmaniosis have been used to assay a variety of experimental vaccines, ranging from whole parasites lysate antigens to DNA vaccines. Formulations using whole parasite lysates have been demonstrating different degrees of protection in both cutaneous and visceral experimental leishmaniosis associated with increasing of cellular immune response [18]. Beside these classical vaccines, a relatively new concept was introduced by Wolff et al. [43]. These researchers used plasmidial DNA to drive the production of several immunizing proteins. This model was immediately transferred to infectious diseases [8] and different Leishmania genes, such as gp63 or LACK, have been linked to bacterial plasmid to produce vaccine candidates [32]. Although important levels of protection have been obtained in animal models, few works were able to demonstrate the mode of action of DNA vaccines [6], which can be a limitation for widely application.
Another interesting source of antigens that can be used to immunize against parasitic diseases are the antigen released/excreted by pathogenic agents [35]. Due to their location or because they are released into the host environment, these antigens are the first to establish a close contact with the immune system of the host modulating its function. Previous studies have demonstrated that antigens secreted by microorganisms can quickly bind to class I and II molecules of major histocompatibility complex priming CD4+ and CD8+ T lymphocytes, while somatic antigens stay encapsulated by cellular membranes, protected from the antigen processing machinery of eukaryotic cells. Only after pathogen destruction the somatic antigens can be processed for cell presentation. Thus, a delay occurs in the stimulation of T cell by this class of antigens [10].
Immunization of mice with L. major secreted antigens decrease skin parasite load and induce a mixed Th1/Th2 immune response [42]. Furthermore, the protective potential of released antigens was also demonstrated in experimental infections caused by L. infantum in both canine and murine leishmaniosis [15, 33] indicating that the pathogenic evolution of Leishmania infection might be profoundly affected by vaccination with this class of antigens. In addition, excreted/secreted antigens are less complex than total leishmanial antigens and can be easily produced and purified, which can favor the identification of their constitution and production of recombinant proteins. For these reasons, released antigens seem to be an interesting target to produce vaccine candidates.
Taken together, the above considerations suggest that released antigens can have a strong potential to induce protection. Therefore, the present study aims to evaluate the protective effect of antigens released to culture medium by L. shawi promastigotes.
Materials and methods
Parasite
Leishmania shawi (MHOM/BR/96/M15789) parasites were isolated from a patient with cutaneous leishmaniosis from the Buriticupu town, Maranhão State, Brazil and identified by monoclonal antibodies and multilocus enzyme electrophoresis at the Evandro Chagas Institute (Belém, Pará State, Brazil). The parasites maintained in BALB/c mice footpad were isolated and grown in RPMI-1640 medium (Gibco Invitrogen, USA) supplemented with 10% heat-inactivated FCS, 0.25 mM HEPES, 10 mg/ml gentamicin and 100 IU/ml penicillin. On the sixth day of culture promastigote forms were centrifuged (1,200×g, 10 min) with phosphate-buffer saline solution (PBS, pH 7.4) and used for mice infection.
Production of antigens released from L. shawi
Stationary phase L. shawi promastigotes (~109 promastigotes) from cultures with two passages in vitro were added to 40 ml of RPMI 1640 protein-free. After 24 h, parasites were counted and washed at 1,000×g, 10 min, 4°C, and the supernatant was collected and immediately concentrated by centrifuging using Amicons filters with a pore of 3 kDa (Millipore, USA). The supernatant were submitted to electrophoresis [12] followed by electroelution [32] of the three main fractions: LsPass1 (>75 kDa), LsPass2 (75–50 kDa) and LsPass3 (<50 kDa). The antigenic fractions were further purified from possible LPS contamination using Detoxi-GelTM Endotoxin Removing Gel (Pierce Biotechnology, USA) according to the manufacturer’s instructions. The protein content of each fraction was quantified in a spectophotometer at 280 nm with correction at 320 nm. The maintenance of L. shawi promastigotes in protein-free medium for such a short time did not affect the viability or the concentration of parasites that suffer a slight increase (~2.0 × 109 promastigotes).
Immunization and challenge
Groups of 10 male BALB/c mice were immunized once a week for two consecutive weeks by subcutaneous route in the rump with 25 μg of LsPass1, LsPass2 or LsPass3 antigenic fraction. Another 10 healthy mice were injected with 50 μl of PBS by the same route, in a similar way. One week after the last immunization, five immunized and five healthy mice were infected intradermally into the hind footpad with 106 stationary phase L. shawi promastigotes (designated as challenged and infected groups, respectively). Five immunized and healthy (non-infected) animals were injected with 50 μl of PBS into the footpad. The infection was monitored weekly for 5 weeks by measuring the size of lesions with a dial micrometer and expressed as the difference in size between the infected and the contra lateral uninfected footpad. Then animals were sacrificed in a CO2 chamber and skin fragments were obtained for parasite burden determination through limiting-dilution assay. Popliteal lymph nodes were collected for determination of parasite load and isolation of CD4+ and CD8+ T cells and double negative (dn) CD4CD8 cells.
In previous studies, our group had verified that after 6 weeks of infection BALB/c mice develop ulcerated lesions with extensive necrosis, usually associated to secondary infections (data not shown). Therefore, to avoid the emerging of extreme clinical situations the experiments were design for a maximum of 5 weeks.
Determination of viable parasite load
The number of viable promastigotes was estimated by limiting dilution assay (LDA) [23]. Briefly, skin fragments and lymph nodes were weighed and homogenized individually in a Schneider 10% FCS. An initial homogenized suspension was placed into the first well (200 μl) and serial dilutions (1:4) were distributed in a 96-multiwell plate (Nunc, Germany). For each sample, 12 replicates were made. After 10 days at 25°C, each well was examined by optical microscopy and the final titer was set as the highest dilution for which the well contained at least one parasite. The viable parasitic load per gram of homogenized organ was calculated as follows: (reciprocal titer of the last positive well per total volume of homogenized organ/tissue × dilution factor) divided by the weight (gram) of the homogenized organ/tissue.
Isolation of lymph node cell subpopulations
CD4+ and CD8+ T cells and dnCD4CD8 cells were isolated from lymph nodes of all groups by positive selection on a magnetic separation system. Lymph nodes were homogenized in RPMI 1640 and the cell number was counted and adjusted to 107 cells/90 μl of elution buffer [PBS + 0.5% bovine serum albumin (BSA, Boehringer, Germany)] with 10 μl of anti-mouse CD4 (L3T4) and CD8 (CD8α) micro beads. After 20 min of incubation cells were washed (300×g, 10 min, 4°C), resuspended in 500 μl of elution buffer and applied to a LS column on magnetic separator (MACS Separator, Miltenyi Biotec, Germany) followed by three washes (500 μl/each) with elution buffer. After the washes, columns were removed from the magnetic separator and CD4+ (purity of 94.1%) or CD8+ T cells (purity of 91.7%) were eluted. dnCD4CD8 cells were collected directly from the LS+column. CD4+ and CD8+ T cells and dnCD4CD8 cells were used for RNA extraction.
RNA extraction, cDNA synthesis and real-time PCR
To evaluate the ex vivo lymphocyte activity the accumulation of cytokines mRNA in CD4+ T, CD8+ T and dnCD4CD8 cell subpopulations freshly isolated was quantified by real-time PCR.
Total RNA was extracted using the RNeasy Mini kit (Qiagen, Germany) according to the manufacturer’s recommendations. The target RNA was reverse transcribed into cDNA using 200U M-MLVRT (Promega, USA), at 37°C for 60 min in the presence of 3 mM 5× M-MLV RT buffer (250 mM Tris–HCl, pH 8.3, 375 mM KCl and 15 mM MgCl2), 10 mM BSA, 1 mM dNTPs (Promega), 40 U rRNAsin ribonuclease inhibitor and 10× Oligo (dT)15 (Promega). The samples were then heated 10 min at 95°C for RT inactivation and cooled to 4°C [15]. Quantitative real-time PCR was performed in the ABI GeneAmp 5700-sequence detection system (Perkin-Elmer/Applied Biosystems, USA). Reaction conditions were powered on a Pentium III Dell Opti Plex GX110 linked directly to the sequence detector. PCR amplifications were performed by using SYBR® Green as a double-strand DNA-specific binding dye with continuous fluorescence monitorization. Amplification was carried out in a total volume of 20 μl containing 2 μl of cDNA sample, 10 μl of 2× SYBR® Green I dye PCR Master Mix [(AmpliTaq Gold® DNA polymerase, dNTP mix with dUTP, passive reference I and optimized buffer components) (Perkin-Elmer/Applied Biosystems)] and primers for HPRT, IFN-γ, TNF-α, IL-4, IL-10 and TGF-β as described by Rosa et al. [33] and Rodrigues et al. [30]. PCR amplification was performed in triplicate wells using the following conditions: 10 min at 95°C for AmpliTaq Gold activation followed by a total of 40 cycles (thermal profile for each cycle: 15 s at 95°C, 1 min at 60°C). To quantify the cytokine expression cDNA plasmid standards for each cytokine and HPRT [30] were used to construct a standard curve in each PCR run.
Statistical analysis
The experiments have been repeated three times and results were expressed as mean ± standard deviation and the non-parametric Mann–Whitney U test was used to compare lesion size, parasite load and cytokine expressions between the groups of mice. Differences were considered statistically significant with a 5% significance level (p < 0.05). Statistical analysis was performed with the SPSS 17.0 for Windows software (SPSS Inc. USA).
Results
Immunization by LsPass2 and LsPass3 confers partial protection to L. shawi infection
During the first 3 weeks, the course of infection was identical in all infected animals, however at 4 and 5 weeks post infection (pi) LsPass2- (p = 0.012; p = 0.023, respectively) and LsPass3-challenged (immunized and infected) mice showed a significant reduction of lesion size (p = 0.018; p < 0.001) when compared with infected mice (Fig. 1).
The course of infection in infected and LsPass1-, LsPass2- and LsPass3-challenged BALB/c mice was accompanied during five weeks. Results are expressed as the mean of lesion sizes determined every week for five consecutive weeks. The experiment was repeated three times. *p < 0.05 indicates statistically significant differences when comparing the lesion size of infected and challenged mice
Furthermore, LsPass2- (p = 0.014) and LsPass3-challenged mice (p = 0.021) presented 72 and 92% lesser parasites, respectively, compared with infected mice. Moreover, LsPass3 antigen was four times more effective than LsPass2 in the induction of protection against L. shawi infection (p < 0.016). The immunization with LsPass1 showed no protection (Fig. 2).
Determination of parasite burden in skin of infected LsPass1-, LsPass2- and LsPass3-challenged BALB/c mice at 5 weeks pi. Parasite load was determined by limiting dilution assay and results are expressed as the mean value of parasites per gram (g) of homogenized skin ±SEM of three independent experiments. *p < 0.05 indicates statistically significant differences when comparing the parasite load of infected and challenged mice
Parasites were not detected in lymph nodes of infected and challenged animals.
Immunization induced the expression of pro-inflammatory cytokines
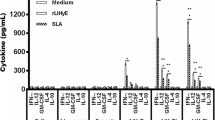
Mice immunized with LsPass1 fraction presented elevated expression of IFN-γ by CD4+ (p = 0.039) and CD8+ T (p = 0.035) cells, however, dnCD4CD8 (p = 0.031) cells showed minimal expression compared to healthy mice. LsPass2-immunized mice had decreased expression of IFN-γ by CD4+ T (p = 0.002) and dnCD4CD8 (p = 0.041) cells. However, CD8+ T cells showed high accumulation of IFN-γ mRNA (p = 0.005). In the group of mice immunized with LsPass3, an elevated expression of this cytokine was only observed in CD4+ T cells (p = 0.023) (Fig. 3a).
Ex vivo mRNA expression of IFN-γ (a), TNF-α (b), IL-4 (c), IL-10 (d) and TGF-β (e) by CD4+ T, CD8+ T and dnCD4CD8 cells of healthy and LsPass1-, LsPass2- and LsPass3-immunized BALB/c mice. The levels of cytokines mRNA were obtained by quantitative real-time PCR and data are mean values ± SEM of three independent experiments expressed as the number of copies per 1,000 copies of housekeeping gene HPRT. *, ** and ***p < 0.05 indicate statistically significant differences when comparing samples from healthy and immunized mice
CD4+ T cells isolated from LsPass1- (p = 0.002) and LsPass3-immunized mice (p = 0.013) expressed high amounts of TNF-α. CD8+ T cells of LsPass2- (p = 0.005) and LsPass3-immunized mice (p = 0.029) revealed increased levels of TNF-α mRNA. On the other hand, dnCD4CD8 cells isolated from lymph nodes of LsPass2-immunized mice showed a reduced expression of TNF-α gene (p = 0.041) (Fig. 3b).
Increases of IL-4 expression was detected in CD4+ (p = 0.008) and CD8+ T cells (p = 0.034) of LsPass1-immunized mice. CD4+ T cells from LsPass2-immunized mice presented elevated expression of IL-4 (p = 0.009) while CD8+ T cells (p = 0.012) expressed reduced amounts of this cytokine. High levels of IL-4 mRNA were accumulated by CD8+ T (p = 0.019) and dnCD4CD8 cells (p = 0.002) of LsPass3-immunized mice whereas CD4+ T cells revealed a significant reduction of this cytokine (p = 0.012) (Fig. 3c).
In LsPass1-immunized mice, CD4+ T cells exhibited a decreased expression of IL-10 (p = 0.023) while CD8+ T cells (p = 0.031) presented high mRNA accumulation. CD8+ T (p = 0.012) and dnCD4CD8 cells (p = 0.023) of LsPass2-immunized mice showed decreased expression of IL-10. CD8+ T cells of LsPass3-immunized mice expressed low amounts of IL-10 mRNA (p = 0.014) whereas dnCD4CD8 cells presented significant high levels of this cytokine (p = 0.032) (Fig. 3d).
In both LsPass1- and LsPass3-immunized mice elevated expression of TGF-β was verified in CD4+ (p = 0.004) and CD8+ T cells (p = 0.027). CD8+ T cells of LsPass2-immunized mice also presented high levels of TGF-β mRNA (p = 0.003), while dnCD4CD8 cells showed minimal expression (p = 0.023) (Fig. 3e).
LsPass3-challenged mice develop a mild pro-inflammatory response
CD4+ T cells of challenged animals presented elevated expression of IFN-γ when compared with infected mice (p LsPass1 and LsPass3 = 0.008; p LsPass2 = 0.009). CD8+ T cells from LsPass2-challenged mice also presented a strong accumulation of IFN-γ mRΝΑ (p = 0.003), although in LsPass1- and LsPass3-challenged mice a significant decrease in the expression of this cytokine was observed (p LsPass1 = 0.006; p LsPass3 = 0.008). dnCD4CD8 cells from both LsPass2- and LsPass3-challenged mice presented a significant high expression of IFN-γ in comparison to infected mice (p LsPass2 = 0.001; p LsPass3 = 0.007) (Fig. 4a).
Ex vivo mRNA expression of IFN-γ (a), TNF-α (b), IL-4 (c), IL-10 (d) and TGF-β (e) by CD4+ T, CD8+ T and dnCD4CD8 cells of infected and challenged BALB/c mice. The levels of cytokines mRNA were obtained by quantitative real-time PCR and data are mean values ± SEM of three independent experiments expressed as the number of copies per 1,000 copies of housekeeping gene HPRT. *, ** and ***p < 0.05 indicate statistically significant differences when comparing samples from healthy and immunized mice
CD4+ T cells isolated from LsPass2- and LsPass3-challenged animals showed a decrease of TNF-α expression when compared with infected mice (p LsPass2 = 0.003; p LsPass3 = 0.046). High expression of this cytokine was also observed in CD8+ T cells of LsPass1- (p = 0.021) and LsPass3-challenged mice (p = 0.019). dnCD4CD8 cells of LsPass3-challenged mice also exhibited a significant accumulation of TNF-α mRΝΑ (p = 0.034) (Fig. 4b)
CD4+ and CD8+ T cells from LsPass2-challenged mice presented high levels of IL-4 mRNA when compared with infected mice (p = 0.008). On the other hand, CD8+ T cells from LsPass1- and LsPass3-challenged animals showed low levels of IL-4 (p = 0.006) (Fig. 4c)
CD4+ T cells of challenged animals presented reduced expression of IL-10 (p = 0.001). However, high levels of IL-10 were observed in CD8+ T and dnCD4CD8 cells of LsPass2-challenged mice. CD8+ T cells of both LsPass- (p = 0.007) and LsPass3-challenged animals (p = 0.003) also revealed low accumulation of IL-10 mRNA (Fig. 4d).
In LsPass1-challenged mice an increasing expression of TGF-β was detected in both CD4+ (p = 0.002) and CD8+ T cells (p = 0.032). LsPass2-challenged mice showed low levels of TGF-β expressed by CD8+ T cells (p = 0.004), while elevated accumulation of this cytokine were observed in CD8+ T cells from LsPass3-challenged mice (p = 0.032) (Fig. 4e).
Discussion
Antigens released from promastigote forms have been identified as potent modulators of the immune system, having a crucial role in promoting the establishment and maintenance of the infection by interfering in the activation of effector mechanisms, such as the microbicidal activity of macrophages and cytokine production. Moreover, interesting studies using released antigens from Leishmania sp. as a model for the development of affordable vaccines have been conducted. Antigens released by L. infantum showed a strong potential to modulate host immunity through a Th1 immune response and induce protection in murine and canine models of zoonotic visceral leishmaniosis [16, 34]. The above considerations support the hypothesis that antigens released by Leishmania species that cause American tegumentar leishmaniosis (ATL) may have potential to induce host protection and be used for vaccine development against these diseases.
The immunization of BALB/c mice with LsPass1 induced overexpression of IFN-γ, IL-4 and TGF-β by both CD4+ and CD8+ T cells. In addition, this immunization increased the levels of TNF-α mRNA by CD4+ T cells and IL-10 by CD8+ T lymphocytes. Therefore, LsPass1 immunized mice presented strong potential to develop cellular immune response and both CD4+ and CD8+ T lymphocytes expressed Th1 cytokines, indicating beneficial response. However, the presence of regulatory mechanism, as measured by IL-10 and TGF-β expression, could be associated to generation of susceptibility since these cytokines induce unresponsive effectors cells [7, 19] leading to low protection. In fact, challenged animals presented lesion size and parasite load similar to infected mice. The absence of protection has been associated to high expression of TGF-β by CD4+ and CD8+ T cells. TGF-β has the capacity to convert CD4+ T lymphocytes to a regulatory phenotype [36, 41], which in turn can depress the effector functions of phagocyte cells [3]. Since TGF-β controls several essential cellular functions a finely tuned regulation of its activity is required. Therefore, it is important to underline that the accumulation levels of TGF-β mRNA does not always correlate with the amount of active protein [41].
Interestingly, in both immunized and challenged groups, the dnCD4CD8 cells (group which contain macrophage, dendritic cell, neutrophil, plasma cells and, natural killer cells) presented an anergic state. Thus, the absence of protection in this group can be associated to both elevation of TGF-β mRNA and suppression of dnCD4CD8.
The LsPass2-immunized mice stimulated mainly CD8+ T cells to express high levels of IFN-γ and TNF-α cytokines. On the other hand, CD4+ T and dnCD4CD8 cells decreased the expression of these cytokines. Increases in the expression of IL-4 by CD4+ and TGF-β by CD8+ T and dnCD4CD8 cells have been observed. In addition, CD8+ T and dnCD4CD8 cells expressed lesser IL-10 than healthy mice. Therefore, the immunization with LsPass2 induced the expression of pro inflammatory cytokines by cytotoxic T cells, which seem to be an important factor of protection in leishmaniosis. In addition, this immunization also induced the expression of regulatory cytokine TGF-β, probably to regulate the exacerbated immune response developed by these animals and to achieve immune homeostasis [4, 9]. After challenge, this group was able to restrain the lesion and parasite load in skin associated to elevated expression of IFN-γ in all cell subsets, indicating that different cells could be activated by immunization such macrophage and natural killer cells [17]. In contrast, CD8+ T cells of these animals have reduced ability to express TNF-α gene, thus the synchronization of different cells subsets to express IFN-γ can favor the protection verified in this group. On the other hand, the level of protection observed in LsPass2-challenged mice can be associated to high accumulation of IL-4 mRNA by CD4+ and CD8+ T cells and of IL-10 mRNA by CD8+ T and dnCD4CD8 cells after vaccination. Therefore, after challenge this group exhibited a mixed Th1/Th2 and Tc1/Tc2 immune response associated to the activation of CD8+ T cell subset with regulator phenotype.
LsPass3-challanged mice were able to enhance the expression of IFN-γ by CD4+ T and TNF-α by CD4+ and CD8+ T lymphocytes, which demonstrates the degree of lymphocyte activation. In comparison with LsPass1- and LsPass2-challenged mice, this group presented lower expression of Th1 and Th2 cytokines possible due to the elevated expression of IL-10 and TGF-β by dnCD4CD8 cells and CD4+/CD8+ T lymphocytes, respectively. This profile of mild expression of both inflammatory and anti-inflammatory cytokines could facilitate the induction of immunity, since this group presented high rate of protection after inoculation of L. shawi promastigotes, associated to decreased lesion size and parasite numbers in skin. Furthermore, after challenged mice still presented a mild Th1 immune response with increased expression of IFN-γ by CD4+ T lymphocytes and dnCD4CD8 cells. The high expression of TNF-α by CD8+ T lymphocytes and dnCD4CD8 cells may be related to phagocyte cells activation and higher levels of protection. Moreover, this group also presented low expression of IL-4 by CD8+T cells and IL-10 was expressed at low levels by both CD4+ and CD8+ T lymphocytes. Beside the impairment of Th2 response and reduced expression of IL-10, the immunization induced the expression of TGF-β by CD8+T cells. Recently, it was also demonstrated that TGF-β restrained the protection induced by immunization with soluble antigen from L. shawi promastigotes even when in presence of a favorable immunological response [29]. Although IL-10 and TGF-β abrogate protection in experimental immunization allowing the persistence of few parasites in the infection site [1], these cytokines avoid immune pathological injuries generated by excessive inflammatory response [31]. In addition, the presence of residual parasites could account for the establishment of a long-lasting immunity conferring a concomitant protection [21].
Infection by parasites of Leishmania subgenus promotes elevated parasitism and low cellular immune response in patients and animals. On the contrary, Leishmania parasites belonging to Viannia subgenus induce low parasitism and high cellular immune response in rodent models and patients [20, 39, 40] suggesting that Viannia antigens are highly immunogenic. In fact, BALB/c mice immunized with antigens derived from L. shawi plus the adjuvant monophosphoryl lipid (MPL) induced IFN-γ associated to a dramatic increase of IL-4 and IL-10 (data not shown). Thereby, additional amplification of cellular immune responses due to the use of adjuvants should also elicit a regulatory and Th2 immune response [5] that may be unfavorable to ATL protection.
Taken together, the results obtained in the present study indicate that antigens release by L. shawi promastigotes to culture medium have stimulatory effects on the host immune system and their pre-exposure to naïve host can prime competent cells to activate macrophages to eliminate intracellular parasites. In spite of conferring high levels of protection, LsPass2 and LsPass3 fractions can be constituted by several molecules, some of which may be counter protective favoring the evasion of the host immune system by the parasite, thus prolonging its survival in host cells due to immunosuppressive antigens [1, 21–26].
Although this study open promising perspectives for the development of a vaccine against ATL further studies are needed to elucidate the role of persistent parasites and regulatory immune response in the maintenance of anti-Leishmania pool of central and effector memory cells. The complete understanding of these factors is crucial for the designing of vaccination strategies against ATL.
References
Bogdan C (2008) Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol 10:1221–1234
Brito ME, Andrade MS, Mendonça MG, Silva CJ, Almeida EL, Lima BS, Félix SM, Abath FG, da Graça GC, Porrozzi R, Ishikawa EA, Shaw JJ, Cupolillo E, Brandão-Filho SP (2009) Species diversity of Leishmania (Viannia) parasites circulating in an endemic area for cutaneous leishmaniasis located in the Atlantic rainforest region of northeastern Brazil. Trop Med Int Health 14:1278–1286
Carneiro FP, De Magalhães AV, De Jesus Abreu Almeida Couto M, Bocca AL, Muniz-Junqueira MI, Ribeiro Sampaio RN (2009) Foxp3 expression in lesions of the different clinical forms of American tegumentary leishmaniasis. Parasite Immunol 31:646–651
Chen G, Han G, Wang J, Wang R, Xu R, Shen B, Qian J, Li Y (2009) Natural killer cells modulate overt autoimmunity to homeostasis in nonobese diabetic mice after anti-CD3 F(ab’)2 antibody treatment through secreting transforming growth factor-beta. Am J Pathol 175:1086–1094
Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, Seder RA (2010) IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med 207:1421–1433
Dunning O (2009) Leishmania vaccines: from leishmanization to the era of DNA technology. Biosci Horiz 2:73–82
Filippi CM, Juedes AE, Oldham JE, Ling E, Togher L, Peng Y, Flavell RA, von Herrath MG (2008) Transforming growth factor-beta suppresses the activation of CD8 + T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes 57:2684–2692
Ivory C, Chadee K (2004) DNA vaccines: designing strategies against parasitic infections. Genet Vaccin Ther 2:17
Kang JS, Liu C, Derynck R (2009) New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol 19:385–394
Kaufmann SH, Hess J (1999) Impact of intracellular location of and antigen display by intracellular bacteria: implications for vaccine development. Immunol Lett 65:81–94
Kreutzer RD, Corredor A, Grimaldi G Jr, Grogl M, Rowton ED, Young DG, Morales A, McMahon-Pratt D, Guzman H, Tesh RB (1991) Characterization of Leishmania colombiensis sp. n (Kinetoplastida: Trypanosomatidae), a new parasite infecting humans, animals, and phlebotomine sand flies in Colombia and Panama. Am J Trop Med Hyg 44:662–675
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lainson R, Braga RR, De Souza AA, Pôvoa MM, Ishikawa EA, Silveira FT (1989) Leishmania (Viannia) shawi sp. n., a parasite of monkeys, sloths and procyonids in Amazonian, Brazil. Ann Parasitol Hum Comp 64:200–207
Lainson R, Shaw JJ (1989) Leishmania (Viannia) naiffi sp. n., a parasite of the armadillo, Dasypus novemcinctus (L.) in Amazonian, Brazil. Ann Parasitol Hum Comp 64:3–9
Lemesre JL, Holzmuller P, Cavaleyra M, Gonçalves RB, Hottin G, Papierok G (2005) Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine 23:2825–2840
Lemesre JL, Holzmuller P, Gonçalves RB, Bourdoiseau G, Hugnet C, Cavaleyra M, Papierok G (2007) Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial. Vaccine 25:4223–4234
Liew FY, Li Y, Millott S (1990) Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol 145:4306–4310
Nagill R, Mahajan R, Sharma M, Kaur S (2009) Induction of cellular and humoral responses by autoclaved and heat-killed antigen of Leishmania donovani in experimental visceral leishmaniasis. Parasitol Int 58:359–366
Naundorf S, Schröder M, Höflich C, Suman N, Volk HD, Grütz G (2009) IL-10 interferes directly with TCR-induced IFN-gamma but not IL-17 production in memory T cells. Eur J Immunol 39:1066–1077
Novoa R, Bacellar O, Nascimento M, Cardoso TM, Ramasawmy R, Oliveira WN, Schriefer A, Carvalho EM (2011) IL-17 and regulatory cytokines (IL-10 and IL-27) in L. braziliensis infection. Parasite Immunol 33:132–136
Okwor I, Uzonna J (2008) Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol Res 41:123–136
O’Daly JA, Lezama R, Rodriguez PJ, Silva E, Indriago NR, Peña G, Colorado I, Gleason J, Rodríguez B, Acuña L, Ovalles T (2009) Antigens from Leishmania amastigotes induced clinical remission of psoriasis. Arch Dermatol Res 301:1–13
O’Daly JA, Lezama R, Gleason J (2009) Isolation of Leishmania amastigote protein fractions which induced lymphocyte stimulation and remission of psoriasis. Arch Dermatol Res 301:411–427
O’Daly JA, Rodriguez B, Ovalles T, Pelaez C (2010) Lymphocyte subsets in peripheral blood of patients with psoriasis before and after treatment with Leishmania antigens. Arch Dermatol Res 302:95–104
O’Daly JA, Gleason JP, Peña G, Colorado I (2010) Purified proteins from Leishmania amastigotes-induced delayed type hypersensitivity reactions and remission of collagen-induced arthritis in animal models. Arch Dermatol Res 302:567–581
O’Daly JA, Gleason J, Lezama R, Rodriguez PJ, Silva E, Indriago NR (2011) Antigens from Leishmania amastigotes inducing clinical remission of psoriatic arthritis. Arch Dermatol Res 303:399–415
Passero LFD, Sacomori JV, Tomokane TY, Corbett CE, da Silveira FT, Laurenti MD (2009) Ex vivo and in vivo biological behavior of Leishmania (Viannia) shawi. Parasitol Res 105:1741–1747
Passero LFD, Marques C, Vale-Gato I, Corbett CEP, Laurenti MD, Santos-Gomes G (2010) Histopathology, humoral and cellular immune response in the murine model of Leishmania (Viannia) shawi. Parasitol Int 59:159–165
Passero LF, Da Costa Bordon ML, De Carvalho AK, Martins LM, Corbett CE, Laurenti MD (2010) Exacerbation of Leishmania (Viannia) shawi infection in BALB/c mice after immunization with soluble antigen from amastigote forms. APMIS 118:973–981
Rodrigues OR, Moura RA, Gomes-Pereira S, Santos-Gomes GM (2006) H-2 complex influences cytokine gene expression in Leishmania infantum-infected macrophages. Cell Immunol 243:118–126
Rodrigues OR, Marques C, Soares-Clemente M, Ferronha MH, Santos-Gomes GM (2009) Identification of regulatory T cells during experimental Leishmania infantum infection. Immunobiology 214:101–111
Rodríguez-Cortés A, Ojeda A, López-Fuertes L, Timón M, Altet L, Solano-Gallego L, Sánchez-Robert E, Francino O, Alberola J (2007) Vaccination with plasmid DNA encoding KMPII, TRYP, LACK and GP63 does not protect dogs against Leishmania infantum experimental challenge. Vaccine 25:7962–7971
Rosa R, Rodrigues OR, Marques C, Santos-Gomes GM (2005) Leishmania infantum soluble proteins released by the parasite exert differential effects on host immune response. Exp Parasitol 109:106–114
Rosa R, Marques C, Rodrigues OR, Santos-Gomes GM (2007) Immunization with Leishmania infantum released proteins confers partial protection against parasite infection with a predominant Th1 specific immune response. Vaccine 25:4525–4532
Santarém N, Silvestre R, Tavares J, Silva M, Cabral S, Maciel J, Cordeiro-da-Silva A (2007) Immune response regulation by Leishmania secreted and nonsecreted antigens. J Biomed Biotechnol 6:85154
Shanmugasundaram R, Selvaraj RK (2010) In vitro human TGF-beta treatment converts CD4(+)CD25(−) T cells into induced T regulatory like cells. Vet Immunol Immunopathol 137:161–165
Shaw JJ, Ishikawa EA, Lainson R, Braga RR, Silveira FT (1991) Cutaneous leishmaniasis of man due to Leishmania (Viannia) shawi Lainson, de Souza, Póvoa, Ishikawa & Silveira, in Pará State, Brazil. Ann Parasitol Hum Comp 66:243–246
Silveira FT, Ishikawa EA, De Souza AA, Lainson R (2002) An outbreak of cutaneous leishmaniasis among soldiers in Belém, Pará State, Brazil, caused by Leishmania (Viannia) lindenbergi n. sp. A new leishmanial parasite of man in the Amazon region. Parasite 9:43–50
Silveira FT, Lainson R, Corbett CE (2004) Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz 99:239–241
Souza-Lemos C, de-Campos SN, Teva A, Côrte-Real S, Fonseca EC, Porrozzi R, Grimaldi G Jr (2008) Dynamics of immune granuloma formation in a Leishmania braziliensis-induced self-limiting cutaneous infection in the primate Macaca mulatta. J Pathol 216:375–386
Taylor AW (2009) Review of the activation of TGF-beta in immunity. J Leukoc Biol 85:29–33
Tonui WK, Mejia JS, Hochberg L, Mbow ML, Ryan JR, Chan AS, Martin SK, Titus RG (2004) Immunization with Leishmania major exogenous antigens protects susceptible BALB/c mice against challenge infection with L. major. Infect Immun 72:5654–5661
Wolff J, Malone R, Williams P, Chong W, Acsadi G, Jani A, Felgner PL (1990) Direct gene transfer into mouse muscle in vivo. Science 1247:1465–1468
Acknowledgments
Funding for this work was provided by the Fundação de Amparo à Pesquisa do Estado de São Paulo (2007/56209-4), LIM50-HCFMUSP and by the Portuguese Foundation for Science and Technology (FCT) with co-participation of the European Union Fund (FEDER) through a research project (PTDC/CVT/70275/2006). This study is part of Luiz Felipe Domingues Passero PhD thesis, supported by Fapesp (07/50654-6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Passero, L.F.D., Marques, C., Vale-Gato, I. et al. Analysis of the protective potential of antigens released by Leishmania (Viannia) shawi promastigotes. Arch Dermatol Res 304, 47–55 (2012). https://doi.org/10.1007/s00403-011-1171-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-011-1171-7