Abstract
Purpose
Differentiating the anatomical variations of the anterosuperior portion of the glenoid labrum from pathologies is important to avoid unnecessary iatrogenic complications resulting from inaccurate diagnosis. Additionally, the presence of several variations was reported to be conductive to lesions involving the glenoid labrum. Thus, the aim of this study was to state the prevalence rates of the sublabral recess, sublabral foramen, and the Buford complex, and to verify their association with labral lesions.
Methods
Systematic search of electronic databases was conducted to gain potentially eligible literature. Suitable studies were selected in a two-round screening, and relevant data were subsequently extracted. Calculation of the pooled prevalence estimates, including sub-analyses on cohort size, study type, and geographical variance, was conducted. Pooled analysis of risk ratios (RR) was used to assess the conductive nature of the discussed variants to superior labrum anterior to posterior (SLAP) lesions.
Results
The screening resulted in selection of 20 studies investigating the morphological features of the glenoid labrum, consisting of 7601 upper limbs. On the bases of random-effects meta-analysis the sublabral recess, sublabral foramen and Buford complex occur with a pooled prevalence of 57.2% (95% CI 30.0–84.4%), 13.5% (95% CI 8.2–18.9%), and 3.0% (95% CI 1.5–4.5), respectively. Moreover, individuals with Buford complex have RR 2.4 (95% CI 1.3–4.7) of developing SLAP lesions, especially type II (95.5%; 95% CI 86.1–100%), whereas such risk for sublabral recess and sublabral foramen was not statistically significant.
Conclusion
Morphological variants of the glenoid labrum posing diagnostic confusion are frequently observed. Gradually, the Buford complex may be a predisposing factor for sustaining a SLAP lesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The glenoid labrum is considered as one of the most important contributors to the static stabilization of the glenohumeral joint [21]. Morphologically, it consists of a peripheral fibrous layer and a transitional fibrocartilaginous layer (zone). Both of these layers provide insertion points for different anatomical structures around the shoulder girdle. Mostly, the peripheral layer serves as an anchor for the tendon of the long head of the biceps brachii muscle as well as for the glenohumeral ligaments and joint capsule. Contrary, the transitional layer provides fine attachment of the aforementioned peripheral layer to the deeper and central parts of the glenoid cavity [13, 20].
Lesions involving the glenoid labrum are common pathological conditions. Especially, injuries of the superior portion of the labrum are nowadays observed with rising incidence in throwing athletes and individuals with a history of fall on an outstretched hand with adducted and flexed arm [18, 22]. These lesions were closely studied by Snyder et al. [22], who called them the superior labrum anterior and posterior (SLAP) lesions since they begin posteriorly and extend anteriorly to the middle part of the glenoid with or without the involvement of the origin of the tendon of the long head of the biceps brachii muscle. Moreover, Snyder et al. [22] classified the SLAP lesions based on their extent as fraying of the superior portion of the labrum with degenerative appearance (type I), tearing with detachment of the origin of the tendon of the long head of the biceps brachii muscle (type II), a bucket-handle displacement with an intact origin of the long head of the biceps brachii muscle (type III), and a bucket-handle fragment extending to the tendon of the long head of the biceps brachii muscle (type IV). Although several extensions have been proposed, the original classification by Snyder et al. [22] is still generally accepted [15].
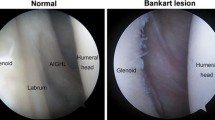
The diagnostic evaluation of the anterosuperior portion of the glenoid labrum remains challenging and requires profound knowledge of the shoulder anatomy [25]. Therefore, the SLAP lesions may be mistaken for normal anatomical variations of the glenoid labrum and vice versa [10, 21]. With heterogeneous prevalence rates the sublabral recess, sublabral foramen, and the so-called Buford complex have been reported to cause diagnostic confusions. The sublabral recess (recessus sublabralis) represents a loose attachment of the superior portion of the labrum on the glenoid cartilage (Fig. 1A) [24, 27]. The sublabral foramen (foramen sublabrale), also termed the sublabral hole, is defined as a window-like structure bordered by the normal anterosuperior labrum and the anterior cartilaginous margin of the glenoid cavity (Fig. 1B) [2, 13, 25]. The Buford complex, first described by Williams et al. [25] is identified upon three criteria: (1) a cord-like middle glenohumeral ligament (MGHL) continuous with anterosuperior portion of the labrum is present; (2) the combined MGHL and anterosuperior portion of the labrum is attached to the superior labrum at the base of the tendon of the long head of the biceps brachii muscle; and (3) no additional anterosuperior labral tissue is present, giving the appearance of a large void below or posteromedial to the cord-like MGHL (Fig. 1C). In addition, it has been reported that the presence of the labral anatomical variations is correlated with higher occurrence of SLAP lesions compared to control groups with regular anatomy [13, 16].
In view of the clinical importance of the anatomical variations observed in the anterosuperior capsulolabral complex, this study aims to create pooled prevalence data on the sublabral recess, sublabral foramen and Buford complex with the use of random-effects meta-analysis, and to verify their conductive attributes to lesions of the glenoid labrum.
Materials and methods
The study protocol was prospectively registered on PROSPERO under the identification number CRD42022356234. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines were consciously followed throughout the study process [17].
Search strategy
A structured search of major scientific databases, including Web of Science, PubMed, ScienceDirect, Scopus, SciELO, JSTOR and Google Scholar, was performed from inception through March 2022. The MeSH terms “Buford complex”, “sublabral foramen”, “sublabral hole”, “foramen sublabrale”, “sublabral recess”, “recessus sublabralis”, “glenoid labrum”, “variability”, and “anatomy” were selected and used in combinations to compile all relevant studies. A representative example of combinations used for Web of Science is attached in Table 1. No restrictions on the language or document type were applied. The reference lists of published articles were also checked for any missed studies.
Data extraction and eligibility criteria
The data were extracted in a two-round screening provided by two reviewing authors (M.B. and V.K.). Citation manager Mendeley v. 1.19.4 (Elsevier, UK) was used for data curation in both rounds of screening. Initially, titles with abstracts were reviewed and all apparently irrelevant hits were excluded. Then, in the second round, the remaining articles were assessed for eligibility by inspecting the full-texts. Only original studies on humans were considered suitable for the meta-analysis. The inclusion criteria met cadaveric studies with natural anatomy, as well as individuals from clinically oriented studies with possible pathological conditions. Review articles, case reports, conference abstracts, studies on animals, and studies containing incomplete or misleading data unresolved by email correspondence were excluded. In case of studies with overlapping data, larger cohort size and relevance of information was the decisive for inclusion. All studies that were not written in English were translated by professionals fluently speaking the particular language. Information on demographics, sample size, year of publication, incidence of the discussed anatomical variations, and eventually the associations between labral morphological variants and labral lesions were extracted. Attempt to contact the original corresponding authors of selected studies was made when uncertainties were recognized during the data extraction.
Quality assessment
Each paper was independently evaluated by two authors (M.B. and V.K.) for risk of bias assessment. Due to the anatomical nature of this meta-analysis the Anatomical Quality Assessment (AQUA) Tool was used as a guide for quality appraisal [8]. This checklist consists of five domains focusing on objectives, study design, methodology, descriptive anatomy, and reporting of results, with each domain having a set of signaling questions. The risk of bias is judged as low if all questions are answered with “Yes”. Contrary, questions answered with “No” might indicate the potential source of bias. All unclear findings were resolved by a reached consensus between all authors. Funnel plots were also used to determine the possibility of bias.
Statistical analysis
The software R v. 4.1.1 (R Project for Statistical Computing, Austria) with RStudio 1.4.1717 (RStudio, USA) was used to analyze all obtained input data. Calculation of pooled prevalence estimates was achieved using the random-effects model. The frequency of the distinct variants and the overall sample size of each included study was used as input data for determining the pooled prevalence estimates. To measure the effect size 95% confidence intervals (95% CI) were calculated. To evaluate the heterogeneity between the included studies Cochran’s Q test and Higgins I2 statistics were used. The P value of < 0.10 was a priori set as “significant heterogeneity”. Values for I2 were categorized as “might not be important” between 0 and 40%, “may indicate moderate heterogeneity” between 30 and 60%, “may represent substantial heterogeneity” between 50 and 90%, and “may represent considerable heterogeneity” between 70 and 100% [9]. Confidence intervals were also used to state statistical significance in terms of their absent overlap when comparing two outcomes. To further investigate the sources of heterogeneity and probe for sources of bias, sub-analyses minding the cohort size, study type, and geographical origin were conducted. Potential of small sample size bias was assessed by visual inspection of funnel plots. Additionally, meta-analysis of binary outcomes was used for inspection of associated pathologies with the morphological variations. Risk ratio (relative risk, RR) was calculated for each of the relevant studies and the individual RRs were consequently pooled for cumulative analysis. Confidence intervals of the calculated RRs were used to determine the statistical significance.
Results
Identification of studies
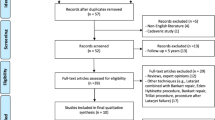
Systematic search of databases yielded a total of 1471 hits, and after checking for duplicates 846 hits remained. In the first round of screening, comprising title and abstract checking, 801 hits were excluded. Full-text screening resulted in 15 studies meeting the inclusion criteria. Additional five studies were added from reference checking. Therefore, a total of 20 studies were finally deemed eligible for inclusion in the meta-analysis. See flowchart displayed in Fig. 1 for detailed overview of the study selection process.
Characteristics of studies
Overall, 20 studies were used for meta-analysis with a total number of upper limbs of 7601 and average cohort of 380.1 cases (range 15–3, 129). Geographically, ten studies originated from North America, five from Asia, four from Europe, and one from Australia. From the total of 20 studies, ten were arthroscopic, six were cadaveric, and four were radiological studies. Among the included studies, twelve of them were evaluated as having high probability of bias according to the AQUA Tool. Study characteristics with risk of bias assessment are summarized in Table 2. Based on the electronic communication with the corresponding authors, the study by Ozer et al. [16] includes patients from the study by Kanatli et al. [13]. Therefore, the study by Kanatli et al. [13] was omitted from the meta-analysis on the Buford complex, but yet this study is included in the analyses regarding the sublabral recess and sublabral foramen since these data do not duplicate with the latter study. Also, study by Ilahi et al. [12] overlaps with a newer study published by the same author in 2008 [11]. The former study was therefore excluded from the analysis (Fig. 2).
Sublabral recess
A total of six studies (896 upper limbs) reported relevant data on the sublabral recess. The analysis revealed that the pooled prevalence estimate is 57.2% (95% CI 30.0–84.4%) (Fig. 3). Both sensitivity analysis and visual inspection of funnel plot indicate that this outcome is biased by the small sample. In general, sub-analyses including studies with larger cohort sizes showed that the actual prevalence might be significantly lower – in extreme situations as low as 2.5% (95% CI 1.3–3.6%) found in an arthroscopic study, which was conducted on a considerable sample. Detailed results are attached in Table 3.
Sublabral foramen
The sublabral foramen was described in 16 studies (2833 upper limbs), and occurs with a pooled prevalence of 13.5% (95% CI 8.2–18.9%). Pooling of the closer morphological findings reported in three studies showed that in 67.2% (95% CI 55.2–79.1%) of cases the foramen existed together with a cord-like MGHL [11, 20, 25]. Throughout the performed sub-analyses the prevalence rates remained nearly identical to the overall analysis with the exception of geographical disparity in Asia and Australia (Table 3). Nevertheless, these sub-analyses include limited sample (Fig. 4).
Buford complex
In total, 19 studies (6910 upper limbs) contained relevant data on the Buford complex. The overall pooled prevalence was calculated to be 3.0% (95% CI 1.5–4.5) (Fig. 5). Higher prevalence rates were found among radiological studies, followed by arthroscopic and cadaveric studies, respectively. Simultaneously, the Buford complex was found more prevalently, although insignificantly, in North America, followed by Asia and Europe, respectively. See Table 3 for detailed results of the performed analyses.
Association with labral pathologies
The association of the anterosuperior capsulolabral variations with SLAP lesions was discussed in four arthroscopic studies (4864 upper limbs) [3, 13, 14, 16]. However, the relationship with sublabral recess and sublabral foramen was observed only in one study [13]. All four studies provided sufficient data for exploring the association of SLAP lesions and the presence of the Buford complex. Conclusively, the sublabral recess and sublabral foramen do not significantly increase the risk of SLAP lesions with RR of 1.4 (95% CI 1.0–1.9) and 1.2 (95% CI 0.9–1.5), respectively (Fig. 6A, B). Conversely, individuals with Buford complex have RR of 2.4 (95% CI 1.3–4.7) of developing a SLAP lesion (Fig. 6C). Most commonly the Buford complex occurs with type II lesions (95.5%; 95% CI 86.1–100%), followed by type I (3.2%; 95% CI 0–10.8%) and type III (< 0.1%; 0–1.9%), respectively.
Discussion
Morphological variations of the anterosuperior capsulolabral complex must be differentiated from lesions of the glenoid labrum and adjacent glenohumeral ligaments. Their relatively common appearance detected in the presented study should be consciously taken in mind during diagnostic shoulder arthroscopies to obtain the correct diagnosis and avoid preventable complications. The presence of the Buford complex may predispose to SLAP lesions. Such outcomes are of particular interest when evaluating the shoulder joint for pathology as the presence of the Buford complex may lead the surgeon towards proper exploration of the anterosuperior portion of the glenoid labrum, where, in particular, type II SLAP lesions occur.
Pooled analysis indicated that the overall prevalence rates for the sublabral recess, sublabral foramen, and Buford complex are 57.2%, 13.5%, and 3.0%, respectively. Nevertheless, further research is required in the field of the sublabral recess since the number of large cohort studies is limited. As the results of the sub-analyses are quite heterogeneous, this probably signifies reporting bias. On the other hand, the equivocal outcomes may also support high variability between research methods or geographical variance. For the reason of insufficient data, the association of sublabral recess and SLAP lesions is only theoretical.
During a shoulder arthroscopy the sublabral recess can resemble a small irregularity of the superior or anterosuperior labral insertion onto the glenoid and therefore can be misdiagnosed as a small labral tear [2, 7]. Compared to SLAP lesions, the sublabral recess is shallow and usually does not extend dorsally over the attachment of the tendon of the long head of the biceps brachii muscle [7, 24]. Nevertheless, extension to the posterior segment of the labrum may occur, which poses diagnostic confusions [24]. Morphologically, the sublabral recess features synovial lining [2]. Performing an excess anterior stabilization of the sublabral recess may limit the patient slightly in his shoulder range of motion.
The sublabral foramen is frequently observed together with the cord-like MGHL (67.2%). Knowledge of this co-incidence is definitively useful when intraoperatively testing the attachment of the glenoid labrum. Nevertheless, the presence of the sublabral foramen was not deemed indicative of pathology as the function of the glenoid labrum is not impaired [24]. This statement is believed to be true in case of intact glenohumeral ligaments and muscles forming the rotator cuff. Only a single study investigating the association of the sublabral foramen and SLAP lesions was identified for our analysis, and the outcomes are insignificant of the conductive nature. The arthroscopic picture of a sublabral foramen can imitate a limited anterior or anterosuperior labral tear (soft Bankart lesion or SLAP lesion), sometimes even forming a communication with the subscapular bursa. Compared to the traumatic lesion, the sublabral foramen is of limited size (up to 1–2 cm), has smoother margins without synovial reaction and is not displaced from the glenoid. A misleading stabilization of this normal labral variant can lead to slight limitation of shoulder range of motion, especially in external rotation.
Conversely, the Buford complex was positively correlated with higher risk of SLAP lesions. The outcomes of our study show that the RR was 2.4 in individuals with Buford complex. Ozer et al. [16] concluded that the risk is raised due to a repetitive micro-trauma and secondary acute trauma explained by increased load bearing on the superior portion of the labrum proximal to the MGHL as the missing anterosuperior portion of the labrum associated with the Buford complex alters physiological distribution of forces applied on the circumferential glenoid labrum. Therefore, the superior labrum is prone to injury due to the applied stress. Compared to the previous variants, the Buford complex can be very convincing during the arthroscopic surgery, resembling severe anterosuperior labral tear (SLAP lesion). With the absence of normal glenolabral insertion between the 1 and 3 o’clock, thickening of the MGHL and direct superior labral insertion onto the subscapularis muscle tendon, it can mimic a major pathology in ventral shoulder chamber. An erroneous anterior stabilization of this fully physiological variant can lead to severe intraoperative damage to anterior joint capsule, incorrect stabilization of subscapularis muscle tendon to anterior part of the glenoid, and produces a severe limitation of shoulder range of motion, especially in abduction, external rotation and dorsal flexion. It belongs to the most debilitating iatrogenic surgical consequences in arthroscopic shoulder surgery.
As mentioned above, individuals with Buford complex have higher risk of developing a SLAP lesion. Therefore, a novel technique modifying the anatomy of the Buford complex in SLAP lesion repairs has been proposed [5]. Apart from the standard SLAP repair, the cord-like MGHL is transected, and the proximal remnant is tightly affixed to the supraglenoid tubercle, while the distal remnant is left intact within the joint. By this change of former morphology, the release of MGHL prevents unwanted pulling on the superior portion of the glenoid labrum and the attachment of the tendon of the long head of the biceps brachii muscle. Performing such a procedure should avoid recurrence of the SLAP lesion in patients with Buford complex, who naturally have higher risk of sustaining this type of injury. From the perspective of intermediate follow-up, this technique has shown satisfactory improvement in outcomes [5]. Significant improvements were reported in motion, pain relief, strength, and subjective satisfaction. Noteworthy, surgical interventions in symptom-free patients with Buford complex should not be performed due to a risk of development of shoulder stiffness [25].
The anatomical variations of our interest have also been discussed as contributing factors to conditions other than the SLAP lesions. Rao et al. [20] compared a group of patients with sublabral foramen occurring with and without the cord-like MGHL, and Buford complex with control group composed of patients with regular anatomy. They found statistically significant association with tears of the tendon of the subscapularis muscle as well as labral fraying. Possible explanation was the increased range of internal rotation in the group of patients with the variants, which predisposes to anterosuperior or coracoid impingement. On the other hand, a significantly lower prevalence of tears of the tendon of the supraspinatus and infraspinatus muscles was observed in the group of patients with abnormal anatomy. This is presumed to be caused by unknown biomechanical alterations that play a protective role. Unfortunately, this study could not be included in our analysis because the data regarding the distinct variants are unextractable for our purposes. However, these findings should encourage researchers to elaborate further on the clinical associations with the intra-articular variations.
Throughout all morphological analyses a high heterogeneity between the data persisted. This can be explained by several aspects. First, a high variance across continents may exist. Second, the methods used to assess the variants feature ranging sensitivity, and thus can be missed especially in the radiological evaluations. And lastly, the limited sample size used for the investigations may not be representative of general population. We believe this is the reason of substantial discrepancy between the sub-analyses performed on the prevalence of the sublabral recess in particular. However, these limiting factors were adjusted in the individual sub-analyses. The arthroscopic studies pose another limitation. It must be borne in mind that the patients underwent arthroscopy due to a symptomatology, and therefore were not randomly selected from the general population. Perhaps, this group may have different prevalence rates of the variants as shown in the sub-analyses minding the study types. To definitively confirm the conductive nature of the Buford complex to sustaining SLAP lesion, prospectively designed observational studies comprising individuals with Buford complex are needed.
Conclusion
Morphological variations in the anterosuperior capsulolabral complex, including the sublabral recess, sublabral foramen and Buford complex, are frequently observed with estimated prevalence of 57.2%, 13.5%, and 3.0%, respectively. These variants must be carefully differentiated from pathological conditions. Moreover, the Buford complex may play a conductive role to SLAP lesions, especially type II. Knowledge of such association should be borne in mind while arthroscopically exploring the shoulder joint for impairments.
Data availability
Data are available on request to corresponding author.
References
Bachler J, Bergman S, Lancigu R, Ropars M, Rony L (2020) Arthroscopic anatomy of the middle glenohumeral ligament. A series of 300 cases. Morphologie 104:187–195. https://doi.org/10.1016/j.morpho.2020.03.002
Bain GI, Galley IJ, Singh C, Carter C, Eng K (2012) Anatomic study of the superior glenoid labrum. Clin Anat 26:367–376. https://doi.org/10.1002/ca.22145
Bents RT, Skeete KD (2005) The correlation of the Buford complex and SLAP lesions. J Shoulder Elbow Surg 14:565–569. https://doi.org/10.1016/j.jse.2005.01.002
Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF (1999) Noncontrast magnetic resonance imaging of superior labral lesions: 102 cases confirmed at arthroscopic surgery. Am J Sports Med 27:208–213. https://doi.org/10.1177/03635465990270021601
Crockett HC, Wingert NC, Wright JM, Bonner KF (2011) Repair of SLAP lesions associated with a Buford complex: a novel surgical technique. Arthrosc 27:314–321. https://doi.org/10.1016/j.arthro.2010.09.005
Handelberg F, Willems S, Shahabpour M, Huskin JP, Kuta J (1998) SLAP lesions: a retrospective multicenter study. Arthrosc 14:856–862. https://doi.org/10.1016/S0749-8063(98)70028-3
Harzmann HC, Burkart A, Wotler K, Vaitl T, Imhoff AB (2003) Anatomische Normvarianten des superioren Labrum-Bizepssehnenanker-Komplexes: anatomische und kernspintomographische Befunde. Orthopade 32:586–594. https://doi.org/10.1007/s00132-003-0488-0
Henry BM, Tomaszewski KA, Ramakrishan PK, Roy J, Vikse J, Loukas M et al (2017) Development of the anatomical quality assessment (AQUA) tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin Anat 3:6–13. https://doi.org/10.1002/ca.22799
Henry BM, Tomaszewski KA, Walocha JA (2016) Methods of evidence-based anatomy: a guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann Anat 205:16–21. https://doi.org/10.1016/j.aanat.2015.12.002
Ide J, Maeda S, Takagi K (2004) Normal variations of the glenohumeral ligament complex: an anatomic study for arthroscopic Bankart repair. Arthroscopy 20:164–168. https://doi.org/10.1016/j.arthro.2003.11.005
Ilahi OA, Cosculluela P, Ho D (2008) Classification of anterosuperior glenoid labrum variants and their association with shoulder pathology. Orthopedics 31:1–4. https://doi.org/10.3928/01477447-20080301-18
Ilahi OA, Labbe MR, Cosculluela P (2002) Variants of the anterosuperior labrum and associated pathology. Arthroscopy 18:882–886. https://doi.org/10.1053/jars.2002.36119
Kanatli U, Ozturk BY, Bolukbasi S (2010) Anatomical variations of the anterosuperior labrum: prevalence and association with type II superior labrum anterior-posterior (SLAP) lesions. J Shoulder Elbow Surg 19:1199–1203. https://doi.org/10.1016/j.jse.2010.07.016
Kaptan AY, Ozer M, Alim E, Percin A, Ayanoglu T, Ozturk BY, Kanatli U (2022) The middle glenohumeral ligament: a classification based on arthroscopic evaluation. J Shoulder Elbow Surg 31:e85–e91. https://doi.org/10.1016/j.jse.2021.07.026
Mohana-Borges AVR, Chung CB, Resnick D (2013) Superior labral anterosuperior tear: classification and diagnosis on MRI and MR arthrography. Am J Roent 181:1449–1462. https://doi.org/10.2214/ajr.181.6.1811449
Ozer M, Kaptan AY, Ataoglu MB, Cetinkaya M, Ayanoglu T, Ince B, Kanatli U (2021) The Buford complex: prevalence and relationship with labral pathologies. J Shoulder Elbow Surg 30:1356–1361. https://doi.org/10.1016/j.jse.2020.08.037
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLOS Med 18:e1003583. https://doi.org/10.1186/s13643-021-01626-4
Pappas ND, Hall DC, Lee DH (2013) Prevalence of labral tears in the elderly. J Shoulder Elbow Surg 22:e11–e15. https://doi.org/10.1016/j.jse.2012.08.023
Park YH, Lee JY, Moon SH, Mo JH, Yang BK, Hahn SH, Resnick D (2000) MR arthrography of the labral capsular ligamentous complex in the shoulder: imaging variations and pitfalls. Am J Roent 175:667–672. https://doi.org/10.2214/ajr.175.3.1750667
Rao AG, Kim TK, Chronopoulos E, McFarland EG (2003) Anatomical variants in the anterosuperior aspect of the glenoid labrum: a statistical analysis of seventy-three cases. J Bone Jt Surg 85A:653–659. https://doi.org/10.2106/00004623-200304000-00011
Shortt CP, Morrison WB, Shah SH, Zoga AC, Carrino JA (2009) Association of glenoid morphology and anterosuperior labral variation. J Comput Assist Tomogr 33:584–586. https://doi.org/10.1097/RCT.0b013e31818da69d
Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ (1990) SLAP lesions of the shoulder. Arthroscopy 6:274–279. https://doi.org/10.1016/0749-8063(90)90056-J
Thompson JM, Carrino JA, Skolasky RL, Chhabra A, Fayad LM, Machado A II, Soldatos T, Morrison WB, McFarland EG (2015) Glenoid notch MRI findings do not predict normal variants of the anterior and superior labrum. Clin Radiol 70:e90–e96. https://doi.org/10.1016/j.crad.2015.04.016
Waldt S, Metz S, Burkart A, Mueller D, Bruegel M, Rummeny EJ, Woertler K (2006) Variants of the superior labrum and labro-bicipital complex: a comparative study of shoulder specimens using MR arthrography, multi-slice CT arthrography and anatomical dissection. Eur Radiol 16:451–458. https://doi.org/10.1007/s00330-005-2864-0
Williams MM, Snyder SJ, Buford D Jr (1994) The Buford complex - the “cord-like” middle glenohumeral ligament and absent anterosuperior labrum complex: a normal anatomic capsulolabral variant. Arthroscopy 10:241–247. https://doi.org/10.1016/S0749-8063(05)80105-7
Wilson WR, Magnussen RA, Irribarra LA, Taylor DC (2013) Variability of the capsular anatomy in the rotator interval region of the shoulder. J Shoulder Elbow Surg 22:856–861. https://doi.org/10.1016/j.jse.2012.08.024
Yeh LR, Kwak S, Kim YS, Pedowitz R, Trudell D, Muhle C, Resnick D (1998) Anterior labroligamentous structures of the glenohumeral joint: correlation of MR arthrography and anatomic dissection in cadavers. Am J Roent 171:1229–1236. https://doi.org/10.2214/ajr.171.5.9798852
Acknowledgements
The authors would like to thank Omer A. Ilahi and Ahmet Y. Kaptan for their correspondence during the data extraction.
Funding
Open access publishing supported by the National Technical Library in Prague. None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Benes, M., Kachlik, D., Kopp, L. et al. Prevalence of the anterosuperior capsulolabral anatomical variations and their association with pathologies of the glenoid labrum: a systematic review and meta-analysis. Arch Orthop Trauma Surg 143, 6295–6303 (2023). https://doi.org/10.1007/s00402-023-04932-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-04932-9