Abstract
Purpose
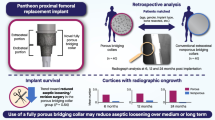
Extracortical osseointegration at the collar-bone interface of megaprostheses is associated with improved implant stability, lower rates of stem fracture and loosening. The use of hydroxy-apatite (HA-) coated collars showed mixed results in previously published reports. A novel collar system has recently become available utilizing additive manufacturing technology to create a highly porous titanium collar with a calcium-phosphate coated surface. The aim of this study was to evaluate our early experience with this novel collar and compare it to the previously used HA-coated model.
Methods
Twenty patients who underwent megaprostheses implantation utilizing the novel collar system were case matched to 20 patients who had previously undergone a HA-coated collar. A minimum radiological follow-up of three months was available in all included patients. Osseointegration was evaluated using postoperative plain radiographs in two planes based on a previously published semi-quantitative score.
Results
Compared to the HA-coated collar the use of the novel highly porous collar was associated with a higher proportion of cases demonstrating osseointegration at the bone-collar interface (80% vs. 65%). Application of the highly porous collar led to a significantly shortened time to reach the final ongrowth score (173 ± 89 days vs. 299 ± 165 days, p < 0.05). At one year follow-up, 90% of the novel collars had reached their final osseoingration grade compared to 50% in the HA-coated collar group (p < 0.001). Radiological osseointegration was seen in 71% for highly porous collars where the indication was revision arthroplasty, compared to 27% in reported in the literature.
Conclusion
These results indicate more reliable and accelerated osseointegration at the bone-collar interface of a novel highly porous collar system compared to a previously used HA-coated collar. Further studies are warranted to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In complex revision arthroplasty and orthopaedic oncology, a commonly used mode of reconstruction is endoprosthetic replacement using megaprostheses [1, 2]. Despite recent advancements in implant design and refinements of implantation techniques resulting in improved prosthesis survival rates, tumour prosthesis remain associated with high complication and revision rates [1, 2]. Available literature demonstrates all-cause revision rates of more than 40% in the first 10 years after implantation [1]. Particularly aseptic loosening was repeatedly reported to be a common mode of failure and cause for revision surgery [2,3,4,5]. One study reported around 30% of patients with tumour prosthesis required at least one revision due to aseptic loosening within 15 years after surgery [1]. Extramedullary osseointegration at the bone-endoprosthetic interface was shown to provide advantageous biomechanical properties thus potentially reducing the risk for aseptic loosening, stem fracture and periprosthetic fractures [3, 6,7,8,9].
In an effort to improve ongrowth capacity at the bone-prosthesis interface different techniques have been employed with mixed results. Aside from bone grafting around the prosthesis shoulder coating of the prosthesis collar with hydroxy-apatite (HA) was utilized [2, 3, 6, 10,11,12]. A recent study observed osseointegration around HA-coated collars in megaprostheses in less than half of reviewed cases [2]. Previous implant failure due to aseptic loosening, arguably the indication that would benefit most from collar osseointegration, was associated with an even worse ongrowth rate with the percentage dropping down to 27% [2]. Evolution of additive manufacturing technology not only allowed for emergence of custom-made patient-specific implants but also facilitated the incorporation of highly complex materials and surfaces in implant design and production processes [13, 14]. Particularly in cases of severe bone loss around the acetabulum but also in complex knee revision arthroplasty highly porous augments and cones, respectively, have recently gained popularity due its usability and reliabilty [14, 15].
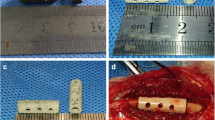
We recently started using a highly porous calcium-phosphate coated EPORE® collar with tumour prostheses (Implantcast, Buxtehude, Germany). EPORE® utilizes tiny rods of Titanium (~ 350 μm) which are arranged together in an open stochastic random pattern to form ‘pores’ of 100–500 μm, similar to that of trabecular bone.
Therefore, the aim of this study was to report on our experience with this novel collar and evaluate the radiological follow-up for evidence of osseointegration.
Methods
Patients
In a first step, we retrospectively identified cases in which the novel 3D-printed collar (EPORE®, Implantcast, Buxtehude, Germany) was utilized. Patient with a radiological follow-up including x-rays in two planes of at least three months after implantation were included in this study. Subsequently, the case matched series of cases of smooth HA-coated collars (MUTARS®, Implantcast, Buxtehude, Germany) with the same requirements on available radiological follow-up was included. Both collars are depicted in Fig. 1. In all cases, fellowship-trained orthopaedic surgeons with longstanding experience in the use of megaprostheses for oncology and revision arthroplasty performed the surgery. The included patients underwent reconstruction utilizing either proximal or distal femur endoprosthetic replacements (MUTARS®, Implantcast, Buxtehude, Germany). Uncemented stems were HA-coated in all cases.
Radiological evaluation of bone ongrowth
Osseointegration was graded using a previously published semi-quantitative scale [2]. Bone ongrowth was assessed by analysing two bone-collar interfaces on the ap (anteroposterior) and lateral x-rays, respectively. The final available set of x-rays was graded. Time to final ongrowth was assessed by identifying the first available postoperative radiographs with the final ongrowth grade. All cases were independently evaluated by two raters and consensus was reached in all cases. Grade 1 represents no visible ongrowth on all four interfaces, grade 2 indicates bony overgrowth with gap formation, grade 3 equals osseointegration in one or two interfaces and grade 4 means visible ongrowth on a least three interfaces [2]. Clinical records were analysed and variables including revision surgeries and complications were collected.
Statistics
Nominal variables between two groups were compared with the Fisher’s exact test, while the chi-square test was utilized for comparison among three or more groups. The Mann–Whitney U-test was utilized to compare continuous variables between two groups. For calculating the time to final ongrowth, the Kaplan–Meier methods was used and curves were compared using the log-rank test. Cases without any radiological evidence for osseointegration were censored at final available follow-up. Spearman’s rank correlation coefficient was calculated for age and ongrowth score as well as age and time to final ongrowth. Age groups with cut-offs of 40 and 65 years were selected for subgroup analysis because they could prove to be potentially clinically relevant. A p value of < 0.05 was considered statistically significant for all employed tests. Data were analysed and visualized using SPSS (Version 25.0, IBM, Armonk, NY, USA) and GraphPad Prism (Version 9, GraphPad Software, La Jolla California USA). Data are given as mean ± standard deviation (SD) if not stated otherwise.
Results
We included 40 patients with 20 patients in each cohort. The mean age was 63 (± 18) years for the entire study population of which 57.5% (n = 23) were male. Detailed demographics for both study groups are depicted in Table 1. There were no statistically significant differences in demographics between the HA-collar and 3D-printed collar group. As we started using the 3D-printed collar only in 2020, the mean follow-up was significantly longer in the HA-coated collar cohort as expected (568 ± 315 vs. 248 ± 125 days, p < 0.001). In all 3D-printed collar cases cement fixation of the stem was used compared to 60% in the HA-collar group (p < 0.01). Indications in both groups included revision arthroplasty, periprosthetic joint infections, primary bone tumours and bone metastases and were comparable among both groups. However, revision for aseptic loosening as indication for surgery was more common in cases where a 3D-printed collar was used (20% vs. 5%, p = 0.34). In each group, there was a single case of stem loosening of a cemented stem due to persisting infection requiring further surgical revision (Table 2).
Overall, in 29 patients (72.5%) radiological evidence for osseointegration was observed with a higher percentage found in the 3D-collar group. This difference, however, did not reach statistical significance (80% vs. 65%, p = 0.48, Fig. 2). There were more grade 1 cases in the HA-collar cohort while grade 3 and grade 4 cases were more prevalent in the 3D-printed collar group (Fig. 3). Patients in the 3D-collar cohort reached their final ongrowth score faster when compared to the HA-collar cohort (173 ± 89 days vs. 299 ± 165 days, p < 0.05). This difference was confirmed in the Kaplan–Meier analysis. At one year follow-up 90% of 3D-printed collars showed final ongrowth score compared to 50% of HA-coated collars (Fig. 4, log-rank test p < 0.001).
We further sought to compare cemented and uncemented stem fixation. In 75% of cemented stems collar osseointegration was observed compared to 62.5% (p = 0.66). Also, time to final ongrowth score was lower in the cemented cohort (217 ± 127 vs. 300 ± 205 days, p = 0.62). However, both differences did not reach statistical significance.
There was no significant correlation between age and final ongrowth score (Spearman’s ρ = 0.238, p = 0.07). Also, when dividing the entire cohort in patients under and above 40 years, no significant differences in ongrowth proportions were found. The same was true when moving the cut-off to 65 years. There was a weak correlation between age and time to final ongrowth indicating reduced time to osseointegration with younger age (Spearman’s ρ = 0.412, p < 0.05). The proportion of cases with osseointegration was not significantly different between cases of revision surgery and primary implantation (77.8% vs. 61.5%, p = 0.239). Also, when comparing proximal and distal femur endoprosthetic replacements no significant difference in percentage of osseointegration was found (65% vs. 80%, p = 0.48). All five cases revised due to aseptic loosening showed ongrowth on at least one bone-collar interface.
Discussion
To the best of our knowledge, this is the first study to report on the osseointegrative capacity of a novel highly porous-coated collars around megaprostheses. These implants are often utilized in highly complex cases of limb reconstruction including revision arthroplasty and oncological cases with an inherent high complication risk. Addition of a highly porous and calcium-phosphate coated collar at the bone-implant interface is thought to facilitate osseointegration. This extracortical bridge between the implant and bone increases implant stability and is able to lower peak forces at the intramedullary site of primary fixation consequently reducing the risk for aseptic loosening and periprosthetic fractures [2, 3, 6,7,8,9]. Also, preventing intraarticular debris from entering the intramedullary bone-implant surface (“purse-string effect”) has been brought forward as potential benefit of additional bone overgrowth at the bone-implant interface [12, 16].
Aseptic loosening was previously shown to be one of the major causes for revision in megaprostheses [1]. This complication was shown to develop particularly within the first five years after surgery [11]. Also, undertaking a revision due to aseptic loosening was reported to be associated with a lower probability of finding radiological evidence for osseointegration [2].
We found two cases (2.5%) of stem loosening, one in each cohort, both on the background of a periprosthetic joint infection requiring further surgical interventions, due to infection. However, no case of aseptic loosening was identified in either group at a mean follow-up of approximately one year.
The indication for the original surgery in five cases (12.5%) was aseptic loosening and in four of these cases the novel 3D-printed collar was utilized. In all five patients, (100%) bone ongrowth at the collar-bone interface was evident radiologically, compared to a reported rate of 27% by Davies et al. This finding potentially indicates that the unique structure of this collar is more likely to provide an osteoconductive and osteoinductive microenvironment even in cases of previous aseptic loosening compared to other collar designs.
In our series, previous revision surgery was not associated with lower rates of osseointegration but, interestingly, rather the opposite was observed with 77.8% osseointegration in previously revised patients compared to 61.5% in primary cases, again in contrast with a previous report from Davies et al. [2] As details regarding the number and indications of previous revision surgeries were not available for this study, a high heterogeneity of these variables might have potentially biassed this result. There were more patients with at least one prior revision in the 3D-printed collar group. Also, with more cases of revision arthroplasty in this group (8 vs. 5 cases) the prerequisites for osseointegration, based on the previous report by Davies et al. were worse compared to the HA-collar group. We argue that these findings further underline the capacity of the 3D-printed collar to induce bone ongrowth even in cases of potentially impaired local biological conditions.
The highly porous structure used in the here evaluated novel 3D-printed collar resembles cancellous bone architecture which aims for mechanically relevant bone ingrowth. The unique microstructure creates a capillary effect at the surface and in combination with calcium-phosphate coating is thought to be particularly instrumental for providing a osteoinductive and osteoconductive milieu [14]. Highly porous surfaces were previously reported to be associated with favourable clinical and radiological outcome in complex reconstruction of the acetabulum and revision knee arthroplasty [14, 15]. But also in megaprostheses porous coating at the implant shoulder was previously associated with high rates of osseointegration and low rates of aseptic loosening. Chao et al. found extracortical bone coverage at the coated implant area of more than 75% on average and less than 5% of cases showed stem loosening [6]. However, their technique involved bone grafting, in most cases autologous graft, around the implant shoulder thus limiting comparability to our series as no local adjuvants were used. Interestingly, cement fixation was associated with lower coverage and ongrowth thickness compared to uncemented fixation in their series [6]. In contrast, cement fixation was associated with a higher rate of osseointegration (75% vs. 62.5%) and reduced time to final ongrowth score in this study. During implantation we paid utmost attention to prevent cement entrapment at the collar-bone interface which might serve as a possible explanation for improved results with cemented stem fixation in our series.
Particularly in uncemented megaprostheses aseptic loosening was shown to develop early, within the first 5 years [11]. The novel 3D-printed collar was associated with an expedited osseointegration when compared to the previously used HA-coated collar (173 ± 89 days vs. 299 ± 165 days, p < 0.05). Therefore, we suggest that particularly in uncemented revision situation where an uncemented stem fixation is preferred or the only option, for instance due to specific anatomical situations or sclerotic endosteal bone on the basis of previous cement fixation, the highly porous collar might prove most beneficial. In our series, most implants were cemented and all prostheses in the 3D-printed collar group were cemented. Chao et al. reported increased bone ongrowth with uncemented stems compared to cement fixation [6].
Follow-up, in general, was limited by the retrospective character of this study. As expected due to later introduction of the 3D-printed collar, mean follow-up was significantly shorter for this cohort. Chao et al. reported that final bone ongrowth was observable at the two-year mark in most cases with only a few included cases showcasing further bone ongrowth beyond this point [6]. Longer follow-up potentially would have further improved results for the novel collar system.
Davies et al. reported a high rate of grade 2, that is overgrowth with an apparent diastasis between new bone formation and the collar, of 41% while only in one patient in our series (2.5%) this pattern was evident [2].
Previous histological workup of explanted megaprostheses revealed novel lamellar bone formation around HA-coated collars while no bone interface in prostheses without collar was reported [3]. On the contrary, Tanzer et al. did not observe bone ingrowth at the porous-coated shoulder of explanted prostheses histologically but fibrous tissue was found which is also capable of providing additional stability [10, 17]. Preclinical studies have shown that additive-manufactured titanium, as also used in the here studied 3D-printed collars, promotes novel bone formation and exhibits biomechanical properties comparable to cancellous bone [18,19,20].
In summary, our findings demonstrate a higher rate of radiological evidence for osseointegration when utilizing a novel 3D-printed collar for megaprostheses. Compared to the HA-coated collar final ongrowth was reached significantly faster. To confirm these results further studies are required.
Limitations
We acknowledge that this study has several limitations. As pointed out by Tanzer et al. reliability of plain radiographs to accurately determine bone ongrowth is questionable [17]. Also, only a semi-quantitative evaluation of collar ongrowth with inherent shortcomings was utilized. However, given the fact that radiological evidence of osseointegration at the bone-collar interface was previously unanimously reported to be associated with lower rates of stem loosening indicates that radiographic evidence for ongrowth is clinically relevant. Also, the available timepoints showed a high inter- and intragroup heterogeneity due to the retrospective design of this study. Heterogeneity of available time points and stem fixation between the two study groups might have influenced the results. Also, smoking was previously demonstrated to negatively impact collar-bone ongrowth. Unfortunately, information about the smoking status of included patients was not available for analysis in this study. Long-term studies will be required to evaluate whether the observed findings translate into favourable outcome in regards to implant survival and revision rates. Also, no functional outcome was available for patients included in this study. Nonetheless, we are confident that novel insights provided by this study are clinically relevant and of interest in view of challenges associated with utilizing megaprostheses.
Data availability
Data will be made available upon request to the corresponding author.
References
Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A (2008) Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Jt Surg Am 90(6):1265–1271
Davies B, Kaila R, Andritsos L, Gray Stephens C, Blunn GW, Gerrand C et al (2021) Osteointegration of hydroxyapatite-coated collars in cemented massive endoprostheses following revision surgery. Bone Jt Open 2(6):371–379
Coathup MJ, Batta V, Pollock RC, Aston WJ, Cannon SR, Skinner JA et al (2013) Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar: a histological study and a radiographic follow-up. J Bone Jt Surg Am 95(17):1569–1575
Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS (1996) Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Jt Surg Br 78(1):5–13
Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H et al (2001) Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res 388:167–177
Chao EY, Fuchs B, Rowland CM, Ilstrup DM, Pritchard DJ, Sim FH (2004) Long-term results of segmental prosthesis fixation by extracortical bone-bridging and ingrowth. J Bone Jt Surg Am 86(5):948–955
Fromme P, Blunn GW, Aston WJ, Abdoola T, Koris J, Coathup MJ (2017) The effect of bone growth onto massive prostheses collars in protecting the implant from fracture. Med Eng Phys 41:19–25
Chao EY, Sim FH (1992) Composite fixation of salvage prostheses for the hip and knee. Clin Orthop Relat Res 276:91–101
Taylor SJ, Perry JS, Meswania JM, Donaldson N, Walker PS, Cannon SR (1997) Telemetry of forces from proximal femoral replacements and relevance to fixation. J Biomech 30(3):225–234
Coathup MJ, Sanghrajka A, Aston WJ, Gikas PD, Pollock RC, Cannon SR et al (2015) Hydroxyapatite-coated collars reduce radiolucent line progression in cemented distal femoral bone tumor implants. Clin Orthop Relat Res 473(4):1505–1514
Batta V, Coathup MJ, Parratt MT, Pollock RC, Aston WJ, Cannon SR et al (2014) Uncemented, custom-made, hydroxyapatite-coated collared distal femoral endoprostheses: up to 18 years’ follow-up. Bone Jt J 96(2):263–269
McDonald DJ (2013) Perspective on hydroxyapatite-coated collars for bone-bridging: commentary on an article by Melanie Jean Coathup, BSc(Hon), PhD, et al.: Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar. A histological study and a radiographic follow-up. J Bone Jt Surg Am 95(17):e1281–e1282
Liang H, Ji T, Zhang Y, Wang Y, Guo W (2017) Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Jt J 99-B(2):267–275
England T, Pagkalos J, Jeys L, Botchu R, Carey SR (2021) Additive manufacturing of porous titanium metaphyseal components: early osseointegration and implant stability in revision knee arthroplasty. J Clin Orthop Trauma 15:60–64
Eachempati KK, Malhotra R, Pichai S, Reddy AVG, Podhili Subramani AK, Gautam D et al (2018) Results of trabecular metal augments in Paprosky IIIA and IIIB defects: a multicentre study. Bone Jt J 100-B(7):903–908
Ward WG, Johnston KS, Dorey FJ, Eckardt JJ (1993) Extramedullary porous coating to prevent diaphyseal osteolysis and radiolucent lines around proximal tibial replacements. A preliminary report. J Bone Jt Surg Am 75(7):976–987
Tanzer M, Turcotte R, Harvey E, Bobyn JD (2003) Extracortical bone bridging in tumor endoprostheses. Radiographic and histologic analysis. J Bone Jt Surg Am 85(12):2365–2370
Imagawa N, Inoue K, Matsumoto K, Omori M, Yamamoto K, Nakajima Y et al (2021) Histological evaluation of porous additive-manufacturing titanium artificial bone in rat calvarial bone defects. Materials (Basel) 14(18):5360
Pattanayak DK, Fukuda A, Matsushita T, Takemoto M, Fujibayashi S, Sasaki K et al (2011) Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Biomater 7(3):1398–1406
Taga T, Kabata T, Kajino Y, Inoue D, Ohmori T, Yamamoto T et al (2018) Comparison with the osteoconductivity and bone-bonding ability of the iodine supported titanium, titanium with porous oxide layer and the titanium alloy in the rabbit model. J Orthop Sci 23(3):585–591
Funding
Open access funding provided by Medical University of Vienna. There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Guy Morris: Consultant for Implantcast (Buxtehude, Germany). Lee M. Jeys: Senior consultant for Implantcast (Buxtehude, Germany), Zimmer Biomet (Warsaw, Indiana, USA), and Stryker (Kalamazoo, Michigan, USA).
Ethical approval
This study was approved by the local institutional review board.
Informed consent
As the study was of retrospective design the need for informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haider, T., Pagkalos, I., Morris, G. et al. Early radiographic osseointegration of a novel highly porous 3D-printed titanium collar for megaprostheses compared to a previous generation smooth HA-coated collar. Arch Orthop Trauma Surg 143, 4671–4677 (2023). https://doi.org/10.1007/s00402-022-04760-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04760-3