Abstract
Background
While continuous optimization is attempted to decrease the incidence of dislocation after total hip arthroplasty (THA), dislocation remains a major complication. This meta-analysis aims to analyze the evolution of the dislocation risk after primary THA over the decades and to evaluate its potential publication bias.
Patients and methods
A systematic search was performed according to the PRISMA guidelines for this meta-analysis in the literature published between 1962 and 2020. MEDLINE, Cochrane and Embase databases were searched for studies reporting the dislocation risk and length of follow-up. Studies that reported on revision rates only and did not mention separate dislocations were excluded. All study designs were eligible. Study quality was assessed by existing quality assessment tools adjusted for arthroplasty research. Overall risk and yearly dislocation rates were calculated and related to historical time frame, study design, sample size and length of follow-up.
Results
In total, 174 studies were included with an overall moderate quality. In total there were 85.209 dislocations reported in 5.030.293 THAs, showing an overall dislocation risk of 1.7%, with a median follow-up of 24 months. The overall dislocation risk classified per decade decreased from 3.7% in 1960–1970 to 0.7% in 2010–2020. The yearly dislocation rate decreased from 1.8 to 0.7% within these same decades. There was no significant correlation between the reported dislocation risk and the duration of follow-up (p = 0.903) or sample size (p = 0.755). The reported dislocation risk was higher in articles with registry data compared to other study designs (p = 0.021).
Conclusion
The dislocation risk in THA has been decreasing over the past decades to 0.7%. Non-selective registry studies reported a higher dislocation risk compared to studies with selective cohorts and RCTs. This indicates that the actual dislocation risk is higher than often reported and ‘real-world data’ are reflected better in large-scale cohorts and registries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hip arthroplasty (THA) is a common orthopedic procedure that has been declared the most successful operation of the twentieth century, being very effective in relieving pain and improving hip function [1]. Due to aging population, the number of primary, and revision THAs is increasing [2]. Surgical techniques and design of the prostheses have been improved since its introduction, in order to optimize clinical outcomes and component survival. A clear example is that various studies demonstrated that larger head size results in a lower risk of dislocation [3,4,5,6,7,8]. However, dislocation remains a major and relatively frequent complication. The majority of the THA dislocations occur in the early postoperative period, but even after years dislocation remains an issue. These late dislocations are mostly a result of wear, precarious movement or an altered spinopelvic mobility [3, 9,10,11,12,13].
At its introduction in 1962, the Charnley ‘low friction’ arthroplasty had a dislocation risk of 4.8% [14]. In 2010, a very large and long-term Medicare cohort study showed a similar dislocation risk of 4.8%, suggesting that the dislocation risk may have not reduced since 1961 [15]. However, studies introducing novel or modified techniques often suggest that these result in a lower risk of dislocation. Unfortunately, those studies often represent a selective cohort and have a relatively short follow-up, retrospective design or small sample.
The aim of this meta-analysis is a thorough evaluation of dislocations rates of primary THAs over the decades and evaluation of the effect of study design, sample size and length of follow-up, hypothesizing a decrease in dislocation risk with a more accurate representation of ‘real-world data’ in large, non-selective cohort and registry studies.
Methods
Search strategy and selection criteria
A systematic search was conducted to collect all research reporting dislocation risk or rates after THA, according to the guidelines of the PRISMA Checklist for reporting systematic reviews and meta-analyses [16]. A search was performed using the MEDLINE, Cochrane Library and Embase databases in December 2020. The search syntax was constructed from the terms ‘total hip arthroplasty,’ ‘dislocation,’ ‘primary’ and its corresponding synonyms in singular and plural. The full search strategy can be found in supplementary data (Supplementary 1). After removal of duplicates, two authors (Author 1 and 2) independently screened articles by title and abstract. Only publications written in English and published after 1962 were considered for review. The full articles were independently screened for eligibility based on predefined inclusion and exclusion criteria. All studies reporting on the dislocation risk of primary THA, with a known duration of follow-up were included. Reviews and meta-analyses were excluded and so were animal studies, studies with < 100 THAs and studies with a follow-up < 1 month. Studies reporting on revision THA because of instability without a separate dislocation rate, resurfacing THA, metal-on-metal THA or dual mobility devices were excluded as well, except for studies reporting dislocation risk of a primary THA population as control group separately. Studies in which the majority of the patients had comorbidities which have a known influence on the dislocation rate were excluded as well, such as Parkinson’s disease, epilepsy, dementia or previous lumbar surgery [3, 4, 10, 17]. In case of multiple studies reporting on a single cohort, only the publication with the longest follow-up was included. Discordant judgments were discussed by two authors until consensus was achieved.
Quality assessment
To assess the quality of the included studies, a checklist with criteria drawn up by Wylde et al. and Evans et al. was used [18, 19]. This checklist is based on existing quality assessment tools for prospective and retrospective studies (MINORs, Newcastle–Ottawa Quality Assessment Scale, ROBINS-I) [20, 21]. Since a relatively high number of patients fails to complete long-term follow-up after THA and several criteria are irrelevant to joint replacement, the checklist was adjusted for THA research. Studies were evaluated for consecutive inclusion, multicenter setting, follow-up of > 80% of the population and the use of multivariate analysis. The individual item ratings were reported as adequate, not adequate or not reported. Ratings of methodological quality of the included studies were conducted by one reviewer and discussed with a second in case of doubt.
Data extraction
The following data were extracted from the included articles: author, year of publication, sample size, years at which the THAs were implanted, mean follow-up, study design, number of THAs and number of dislocated THAs during the study. Only studies which described the percentage or actual number of dislocations were included. Studies that only described a revision rate were excluded, since a considerable part of the patients experience dislocation only without need for revision [3, 22, 23]. Data on surgical techniques and patient factors were not extracted because they were not available, or not comparable in a lot of studies.
The overall dislocation risk was calculated by the total number of dislocated THAs from all studies and divided by the total number of THAs. The studies were categorized by decade, based on the year in which the first surgeries were performed. The overall dislocation risk was calculated for each decade and analyzed continuously. Yearly dislocation rates were calculated by dividing the dislocation rate by the mean follow-up time. Studies with a follow-up less than one year were excluded for these analyses. The effect of the length of follow-up and sample size on the reported dislocation risk were analyzed as a continuous parameter. In addition, we calculated the overall dislocation rate and yearly risk for the different study designs and for studies with and without ‘real-world data’ (RWD). This was defined as studies with a minimum of 1000 patients and 1-year follow-up.
Statistical analysis
The data were assessed using R 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria). Statistical packages used were psych, meta and metaphor. All graphs were made using the ggplot2 package [24]. Heterogeneity among studies was quantified by the I-square and tested using Cochran’s Chi-square tests. Normality was assessed by comparing mean to median and by testing for skewness and kurtosis using Shapiro test. Funnel plots were used to explore possible asymmetry or an unequal distribution of studies. Reported dislocation rates were compared using the Mann–Whitney test and the Kruskal–Wallis test, and p < 0.05 was considered statistically significant. Additionally, multivariate meta-regression analysis was used to determine the influence of follow-up duration on the reported dislocation risk, after adjusting for design sample size and decade.
Results
Search results
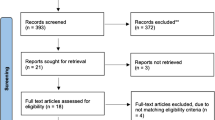
Our search yielded a total of 2383 publications. After applying the in- and exclusion criteria to full-text articles, a total of 170 unique publications remained (Fig. 1). Three studies included cohorts that covered more than one decade, and one study studied both a short-term and a long-term cohort. These four were included as separate series in the statistical analysis, resulting in a total of 174 THA populations. Five studies were RCTs, 32 registry studies (studies using national or regional implant/health/insurance registers), 46 prospective and 91 retrospective cohort studies 97 (45.4%) studies reported on > 1000 THAs. The mean length of follow-up was 36 months, with a median of 24 months (range 1–192).
The quality of included studies was moderate. The assessment showed that 78 (44.9%) studies were consecutive, 39 (22.9%) multicenter, 90 (52.9%) had less than 20% loss to follow-up and 53 (31.2%) used multivariable analyses. (Table 1 Supplementary). None of the overall dislocation rates and risks were normally distributed and therefore described using median and interquartile range (IQR).
Overall dislocation risk
The included studies involved a total of 5.030.293 THAs, of which 85.209 had dislocated, resulting in an overall dislocation risk of 1.7% (Table 1). The yearly dislocation rate was on average 1.0% per year of follow-up.
Historic comparison
A decline in reported dislocation risk was observed over the past 50 years (p < 0.001) (Tables 1 and 2, and Fig. 2): The overall dislocation risk declined from 3.7 to 0.7% from 1962 to 2020. The median follow-up and the yearly dislocation rate also decreased, but not significantly, from 37.1 to 12.5 months and from 1.8 to 0.7%, respectively. The greatest decline in overall dislocation risk was observed from 1970 to 1980. After adjustment for confounders (follow-up, sample size and design), each consecutive year the overall dislocation risk decreased 4.7% relatively to the previous year (estimate = − 0.047; 95% CI − 0.062 − 0.032; p < 0.001).
Risk of publication bias in reporting of THA dislocations
In Fig. 3, the dislocation risk is shown according to follow-up duration, and in Fig. 4, this is subdivided for each decade. The overall dislocation risk was higher when the follow-up duration was longer. However, this difference was not statistically significant (p = 0.903). Table 3 shows the overall risk and yearly dislocation rate for different study designs, decades and studies with and without RWD. In studies with RWD, the overall risk and yearly rate were 2.1 and 1.0%, respectively, whereas those were 1.4 and 1.1% in studies without RWD.
Table 4 shows the multivariate meta-regression analysis for follow-up period, sample size, year in which the study started and study design. While study design was an independent predictor for the dislocation risk, length of follow-up and sample size were not (p = 0.903 and p = 0.755, respectively). Registry studies reported significantly higher dislocation rates (estimate 0.573; 95% CI 0.087–1.060; p = 0.021) compared to other designs after adjusting for follow-up period, sample size and year in which the study started.
Discussion
The aim of this meta-analysis was a thorough analysis of the evolution of the dislocations risk of primary THAs over the past decades and evaluation of its potential publication bias. Over the last 50 years, the overall dislocation risk was 1.7%; however, it declined from 3.7% in the 1970s to 0.7% the last decade. Analysis of the yearly dislocation rates demonstrated a decrease in improvement over the decades. Investigators from the Mayo clinic described a cumulative dislocation risk in a series of more than 6000 THAs of 1.9% after 1 month and 1.8% at 1 year, which are comparable to our results. The risk rose at a constant 1% for every additional 5 years of follow-up, reaching 7% at 20 years. The risks were greater in older patients (> 70 years old) and in females [25].
The decrease in dislocation risk could be a result of improvement in surgical techniques, such as muscle sparing, alternative approaches, increased head sizes and improved implant design [26, 27]. Optimized implant positioning with restauration of femoral offset contributes to THA stability as well [28, 29]. Recent studies have confirmed the importance of capsular repair; optimal stability is acquired with transosseous bone sutures, instead of capsular resection [30, 31]. No difference was found between absorbable and non-absorbable sutures for this repair [32]. Improved recognition by surgeons of patient-related risk factors for dislocation might also be contributing.
However, patient-related risk factors, as well as patient selection have changed over time [33, 34]. Therefore, it can be argued, that improvements in dislocation rates are not the result of technical and surgical improvements alone. Performing surgery on more females with extremes of age, with a higher BMI or patients with a higher level of frailty or spinal stiffness, without a long-term follow-up might miss out on late dislocations [4, 13, 26, 33,34,35,36,37]. Broadening of the indication for THA such as THA placement for pathological fractures or acetabular or femoral neck fractures might contribute as well [38,39,40]. This causes an overall decline in dislocation rate, but a stable yearly dislocation rate.
Reported complication rates are also influenced by the quality of reporting in the THA literature. Our analysis shows that no decrease of dislocation rates have been made since the 1980s. Additionally, due to a time-lag bias in limited follow-up of studies performed in the latest decade, the dislocation rate might actually be increasing. The decline in follow-up over the decades combined with a change in patient population could have attributed to this.
Furthermore, despite the improved methods for registration of dislocations, such as digitalization, (national) implant registries and international scientific society databases, we believe that the reported risk and rate of dislocation is still an underestimation of the true prevalence and incidence. As a result of attrition bias, thus patients tending to move, migrate or being transferred to other hospitals, not all dislocations will be reported. This is confirmed by the finding that the reported dislocation risk is not related to the length of follow-up in the meta-regression analysis. This can be explained by a median follow-up that was relatively short (24 months) in all studies, with a low proportion of studies with > 10-year follow-up. The high dislocation risk in studies with > 10-year follow-up in the 2000s (Fig. 3) demonstrates the reporting bias introduced when long-term dislocations are not included. It can be expected that early dislocations are often registered as complications, while late dislocations might not be registered. Late dislocations are often caused by implant wear and/or by patient-specific changes, such as alterations in spinopelvic alignment or soft tissue atrophy, which can become clinically relevant years after THA implantation [11, 12].
Devane et al. retrospectively studied the true dislocation rates of their hospital compared to the rates in the national registry of New Zealand [41]. They found that revisions were recorded correctly, but patients who were treated with a closed reduction only (57%), were often not identified. Similarly, a Danish study from Hermanssen et al. also showed a lower registration of dislocations in the national register compared to the true rate, and only 50% of the true incidence was identified [42]. Since part of the dislocations remain stable after the first dislocation, the true dislocation rate will be underestimated [3, 22, 23]. For this reason, in this review only registry studies that reported all dislocations, and not only revisions, were included. In a large registry study by Bozic et al. a 3.7% revision rate due to instability (48.217 revisions due to instability in 1.300.666 THAs) was reported [43]. To determine the true dislocation risk in THA, registries should also include dislocations for which no revision surgery was performed. Over time evolving patient-, surgery- and implant-related confounders should be included as well and should be the focus of future research, as part of the post-market surveillance. This might lead to continuous monitoring of the real dislocation rate and can be used to improve clinical outcomes and patient information.
The guidelines of many orthopedic journals classify RCTs as the highest level of evidence. RCTs are designed to avoid bias, but are not necessarily the best design for collection of epidemiological data on surgical complications. Even when randomization and blinding are successful, and crossover of patients is adequately dealt with, the results will often be limited to a selected subgroup and depending on surgical experience of selected surgeons. In 1996, Black et al. discussed the RCT’ low external validity: RCTs are designed to test for efficacy, but are only representative for very specific patients, because of the strictly controlled test population [44]. RWD can only be obtained by very large observational studies and registries with sufficient follow-up [44,45,46]. Registry studies also have disadvantages: a limited availability of patient data and follow-up, no control population and possible duplications or wrong inclusion of patients because of anonymity and miscoding. However, a meta-analysis of Abraham (2010) showed that results of well-designed non-randomized studies, including registries, are just as accurate as that from RCTs [47]. Furthermore, in general, RCTs and smaller cohort studies are often contract research, as compared to investigator or society initiated research in large-scale cohorts. In our study was the reported dislocation risk higher in the large-scale cohorts and registry studies (RWD), compared to the other studies. The reported yearly dislocation rates, however, were comparable between studies with and without RWD.
The data from this thorough meta-analysis show that, for the purpose of reflecting the actual dislocation risk, data from large-scale cohorts and registries are superior to the data obtained from medium-size RCTs. It is likely that the higher dislocation risk reported in studies with RWD is more accurate than the non-RWD and underreporting is the general weakness. Therefore, these non-randomized large-scale cohorts and registries should be considered the highest level of evidence in this type of surgical research.
Because of the enormous heterogeneity and lack of reporting in many studies, confounders such as patient-related factors, for example body mass index and American Society of Anaesthesiologists score, population characteristics, surgical technique and approach and implants used, were not taken into account. This can be considered a limitation of this review, and no conclusions on the effect of individual technical improvements on the dislocation risk can be drawn. In this review, cohort studies or RCTs with relatively small sample size (n < 100) were not included, because of the risk of selection bias and the low incidence of dislocations.
Conclusion
The overall dislocation risk is 1.7% after primary THA. The overall dislocation risk in THA has been decreasing from 3.7 to 0.7%, with no improvement in the yearly dislocation rate. We found no significant reporting bias on the dislocation rates regarding sample size and follow-up duration. Articles with registry data reported a higher dislocation risk compared to other study types. This indicates underreporting of dislocations in RCTs and smaller cohort studies. Therefore, large-scale cohorts and registries should be seen as a more accurate representation of real-world data.
Data availability
Not applicable.
Code availability
Not applicable.
References
Learmonth ID, Young C, Rorabeck C (2007) The operation of the century: total hip replacement. Lancet 370:1508–1519. https://doi.org/10.1016/S0140-6736(07)60457-7
Bozic KJ, Kurtz SM, Lau E et al (2009) The epidemiology of revision total hip arthroplasty in the united states. J Bone Jt Surg 91:128–133. https://doi.org/10.2106/JBJS.H.00155
Sanchez-Sotelo J, Berry DJ (2001) Epidemiology of instability after total hip replacement. Orthop Clin North Am 32:543–552. https://doi.org/10.1016/S0030-5898(05)70225-X
Dudda M, Gueleryuez A, Gautier E et al (2010) Risk factors for early dislocation after total hip arthroplasty: a matched case-control study. J Orthop Surg (Hong Kong) 18:179–183. https://doi.org/10.1177/230949901001800209
Lombardi AV, Skeels MD, Berend KR et al (2011) Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop Relat Res 469:1547–1553. https://doi.org/10.1007/s11999-010-1605-0
Jameson SS, Lees D, James P et al (2011) Lower rates of dislocation with increased femoral head size after primary total hip replacement: a 5-year analysis of NHS patients in England. J Bone Jt Surg—Ser B 93-B:876–880. https://doi.org/10.1302/0301-620X.93B7.26657
Kostensalo I, Junnila M, Virolainen P et al (2013) Effect of femoral head size on risk of revision for dislocation after total hip arthroplasty. Acta Orthop 84:342–347. https://doi.org/10.3109/17453674.2013.810518
Neupane G, Madhusudhan R, Shrestha A, Vaishya R (2020) Large diameter head in primary total hip arthroplasty: a systematic review. Indian J Orthop 54:784–794. https://doi.org/10.1007/s43465-020-00146-y
Bedard NA, Martin CT, Slaven SE et al (2016) Abnormally high dislocation rates of total hip arthroplasty after spinal deformity surgery. J Arthroplasty 31:2884–2885. https://doi.org/10.1016/j.arth.2016.07.049
Buckland AJ, Puvanesarajah V, Vigdorchik J et al (2017) Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Jt J 99B:585–591. https://doi.org/10.1302/0301-620X.99B5.BJJ-2016-0657.R1
Heckmann N, McKnight B, Stefl M et al (2018) Late dislocation following total hip arthroplasty: spinopelvic imbalance as a causative factor. J Bone Jt Surg—Am 100:1845–1853. https://doi.org/10.2106/JBJS.18.00078
Snijders TE, Schlösser TPC, Heckmann ND et al (2021) The effect of functional pelvic tilt on the three-dimensional acetabular cup orientation in total hip arthroplasty dislocations. J Arthroplasty 36:2184-2188.e1. https://doi.org/10.1016/j.arth.2020.12.055
van der Gronde BATD, Schlösser TPC, van Erp JHJ et al (2022) Current evidence for spinopelvic characteristics influencing total hip arthroplasty dislocation risk. JBJS Rev. https://doi.org/10.2106/JBJS.RVW.22.00038
Charnley J (1961) Arthroplasty of the hip: a new operation. Lancet 277:1129–1132. https://doi.org/10.1016/S0140-6736(61)92063-3
Malkani AL, Ong KL, Lau E et al (2010) Early- and late-term dislocation risk after primary hip arthroplasty in the medicare population. J Arthroplasty 25:21–25. https://doi.org/10.1016/j.arth.2010.04.014
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. https://doi.org/10.1136/bmj.b2700
Woolson ST, Rahimtoola ZO (1999) Risk factors for dislocation during the first 3 months after primary total hip replacement. J Arthroplasty 14:662–668. https://doi.org/10.1016/S0883-5403(99)90219-X
Wylde V, Beswick AD, Dennis J, Gooberman-Hill R (2017) Post-operative patient-related risk factors for chronic pain after total knee replacement: a systematic review. BMJ Open 7:e018105
Evans JT, Evans JP, Walker RW et al (2019) How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet (London, England) 393:647–654. https://doi.org/10.1016/S0140-6736(18)31665-9
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73:712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Sterne JAC, Hernán MA, McAleenan A et al. (2019) Assessing risk of bias in a non-randomized study. In: Cochrane Handb Syst Rev Interv. https://www.training.cochrane.org/handbook/current/chapter-25%0A. https://www.training.cochrane.org/handbook/current/chapter-25#section-25-6. Accessed 23 Jan 2021
Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ (2006) Hospital cost of dislocation after primary total hip arthroplasty. J Bone Jt Surg 88:290–294. https://doi.org/10.2106/JBJS.D.02799
Kotwal RS, Ganapathi M, John A et al (2009) Outcome of treatment for dislocation after primary total hip replacement. J Bone Jt Surg - Ser B 91:321–326. https://doi.org/10.1302/0301-620X.91B3.21274
Valero-Mora PM (2010) ggplot2: elegant graphics for data analysis. Springer, Berlin
Berry DJ, Von Knoch M, Schleck CD, Harmsen WS (2004) The cumulative long-term risk of dislocation after primary charnley total hip arthroplasty. J Bone Jt Surg 86:9–14. https://doi.org/10.2106/00004623-200401000-00003
Rowan FE, Benjamin B, Pietrak JR, Haddad FS (2018) Prevention of dislocation after total hip arthroplasty. J Arthroplasty 33:1316–1324. https://doi.org/10.1016/j.arth.2018.01.047
Berry DJ, Von Knoch M, Schleck CD, Harmsen WS (2005) Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am 87:2456–2463. https://doi.org/10.2106/JBJS.D.02860
Vigdorchik JM, Sharma AK, Elbuluk AM et al (2021) High Offset stems are protective of dislocation in high-risk total hip arthroplasty. J Arthroplasty 36:210–216. https://doi.org/10.1016/J.ARTH.2020.07.016
Peng L, Zeng Y, Wu Y et al (2021) Radiologic restoration inaccuracy increases postoperative dislocation in primary total hip arthroplasty: a retrospective study with propensity score matching. Arch Orthop Trauma Surg. https://doi.org/10.1007/S00402-021-04263-7
Dimentberg E, Barimani B, Alqahtani M et al (2022) The incidence of hip dislocation after posterior approach primary total hip arthroplasty: comparison of two different posterior repair techniques. Arch Orthop Trauma Surg. https://doi.org/10.1007/S00402-022-04609-9
Kobayashi N, Kamono E, Kameda K et al (2022) Is there any clinical advantage of capsular repair over capsular resection for total hip arthroplasty? An updated systematic review and meta-analysis. Arch Orthop Trauma Surg. https://doi.org/10.1007/S00402-022-04444-Y
Edipoglu E (2021) Durability of transosseous repair of posterior soft tissues after primary total hip arthroplasty: a prospective randomized controlled trial. Arch Orthop Trauma Surg. https://doi.org/10.1007/S00402-021-04118-1
Pirruccio K, Sloan M, Sheth NP (2019) Trends in obesity prevalence among total hip arthroplasty patients and the effect on surgical outcomes, 2008–2016. J Orthop 16:347–352. https://doi.org/10.1016/J.JOR.2019.03.024
Johnson CA, White CC, Kunkle BF et al (2021) Effects of the obesity epidemic on total hip and knee arthroplasty demographics. J Arthroplasty 36:3097–3100. https://doi.org/10.1016/J.ARTH.2021.04.017
Huddleston JI, Wang Y, Uquillas C et al (2012) Age and obesity are risk factors for adverse events after total hip arthroplasty. Clin Orthop Relat Res 470:490–496. https://doi.org/10.1007/s11999-011-1967-y
Johnson RL, Abdel MP, Frank RD et al (2019) Impact of frailty on outcomes after primary and revision total hip arthroplasty. J Arthroplasty 34:56-64.e5. https://doi.org/10.1016/J.ARTH.2018.09.078
Sychterz CJ, Engh CA, Yang A, Engh CA (1999) Analysis of temporal wear patterns of porous-coated acetabular components: Distinguishing between true wear and so-called bedding-in. J Bone Jt Surg 81:821–830. https://doi.org/10.2106/00004623-199906000-00009
Boddapati V, Held MB, Levitsky M et al (2021) Risks and complications after arthroplasty for pathological or impending pathological fracture of the hip. J Arthroplasty 36:2049-2054.e5. https://doi.org/10.1016/J.ARTH.2021.02.004
Jauregui JJ, Weir TB, Chen JF et al (2020) Acute total hip arthroplasty for older patients with acetabular fractures: a meta-analysis. J Clin Orthop trauma 11:976–982. https://doi.org/10.1016/J.JCOT.2020.01.003
Lewis DP, Wæver D, Thorninger R, Donnelly WJ (2019) Hemiarthroplasty vs total hip arthroplasty for the management of displaced neck of femur fractures: a systematic review and meta-analysis. J Arthroplasty 34:1837-1843.e2. https://doi.org/10.1016/J.ARTH.2019.03.070
Devane PA, Wraighte PJ, Ong DCG, Horne JG (2012) Do joint registries report true rates of hip dislocation? Clin Orthop Relat Res 470:3003–3006. https://doi.org/10.1007/s11999-012-2323-6
Hermansen LL, Viberg B, Hansen L, Overgaard S (2021) “True” cumulative incidence of and risk factors for hip dislocation within 2 years after primary total hip arthroplasty due to osteoarthritis: a nationwide population-based study from the danish hip arthroplasty register. J Bone Joint Surg Am 103:295–302. https://doi.org/10.2106/JBJS.19.01352
Bozic KJ, Kamath AF, Ong K et al (2015) Comparative epidemiology of revision arthroplasty: failed THA poses greater clinical and economic burdens than failed TKA. Clin Orthop Relat Res 473:2131–2138. https://doi.org/10.1007/s11999-014-4078-8
Black N (1996) Why we need observational studies to evaluate the effectiveness of health care. Br Med J 312:1215–1218. https://doi.org/10.1136/bmj.312.7040.1215
Beks RB, Bhashyam AR, Houwert RM et al (2019) When observational studies are as helpful as randomized trials: examples from orthopedic trauma. J Trauma Acute Care Surg 87:730–732. https://doi.org/10.1097/TA.0000000000002347
Houwert RM, Beks RB, Dijkgraaf MGW et al (2021) Study methodology in trauma care: towards question-based study designs. Eur J Trauma Emerg Surg 47:479–484. https://doi.org/10.1007/s00068-019-01248-5
Abraham NS, Byrne CJ, Young JM, Solomon MJ (2010) Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol 63:238–245. https://doi.org/10.1016/j.jclinepi.2009.04.005
Author information
Authors and Affiliations
Contributions
ThS and JvE designed the study. JvE and MH searched and included the articles and extracted the data. MF and JvE carried out statistical analysis. MH, JvE and MF conducted the analysis. JvE, MH and TS drafted the manuscript. TS, AdG, MK and ThS critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Van Erp, Hüsken, Filipe, Kruyt, Schlösser, Snijders and De Gast declare that there is no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
All authors agreed to participate.
Consent for publication
All authors are in agreement with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Erp, J.H.J., Hüsken, M.F.T., Filipe, M.D. et al. Did the dislocation risk after primary total hip arthroplasty decrease over time? A meta-analysis across six decades. Arch Orthop Trauma Surg 143, 4491–4500 (2023). https://doi.org/10.1007/s00402-022-04678-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04678-w