Abstract
Introduction
Adolescent idiopathic scoliosis (AIS) affects 1–3% of the population, but its pathogenesis remains unclear. The coexistence of musculoskeletal hypermobility and scoliosis in many inherited syndromes raises the possibility that isolated musculoskeletal hypermobility may contribute to AIS development or progression.
Methods
We performed a systematic review of the evidence for a relationship between isolated musculoskeletal hypermobility and AIS. A meta-analysis was planned, but if not possible, a narrative evidence synthesis was planned.
Results
Nineteen studies met eligibility criteria for inclusion. One study was excluded due to insufficient quality. Substantial heterogeneity in study design and methodology negated meta-analysis, so a narrative review was performed. Of the 18 studies included, seven suggested a positive association and eight found no association. Three reported the prevalence of musculoskeletal hypermobility in individuals with AIS. Overall, there was no convincing population-based evidence for an association between musculoskeletal hypermobility and AIS, with only two case–control studies by the same authors presenting compelling evidence for an association. Although populations at extremes of hypermobility had a high prevalence of spinal curvature, these studies were at high risk of confounding. Wide variation in methods of measuring musculoskeletal hypermobility and the challenge of assessing AIS in population-based studies hinder study comparison.
Conclusions
There is a paucity of high-quality evidence examining the association between isolated musculoskeletal hypermobility and AIS. Large-scale prospective studies with adequate adjustment for potential confounding factors could clarify the relationship between musculoskeletal hypermobility and AIS to elucidate its role in the pathogenesis of AIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scoliosis is a lateral and rotational deformity of the spine. The most common type is adolescent idiopathic scoliosis (AIS), which presents after age 10 and has a prevalence between 1 and 3% [1]. Even small curves are associated with back pain both in adolescence and later life [2], and scoliosis can have considerable psychosocial impacts, particularly on body image [3]. At extremes, scoliosis can affect respiratory function [4], and severe and progressive AIS can require extensive surgery.
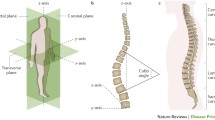
The pathogenesis of AIS remains unclear, but is most likely to be multifactorial. Factors including greater height, delayed puberty and late menarche in females, and low BMI have been shown to contribute [5,6,7]. The gold standard for diagnosis of AIS is via antero-posterior radiography, with a measurement of the Cobb angle of the scoliotic curve over 10° diagnostic of AIS [8]. Screening methods include the Adam’s forward bend test (FBT), with scoliometer measurement of the angle of trunk rotation (ATR) increasing sensitivity and specificity, and back surface topography, which uses contours visible on digital images to detect spinal deformity.
Musculoskeletal hypermobility is common, with a wide variation in prevalence, reported between 7% and 59% in adolescents [9,10,11]. It is more common in females and generally reduces with age [12]. It exists on a spectrum, ranging from asymptomatic hypermobility through to hypermobility spectrum disorder, with associated symptoms including joint clicking and musculoskeletal pain [13]. The most commonly used measure of hypermobility is the Beighton score, which assesses the mobility of nine joints [14]. Traditionally, a score of ≥ 4/9 hypermobile joints signifies generalised musculoskeletal hypermobility, although this cut-off may over-represent clinically important musculoskeletal hypermobility [15].
Both musculoskeletal hypermobility and scoliosis are features of inherited syndromes including Marfan’s syndrome, osteogenesis imperfecta and certain types of Ehlers-Danlos syndrome, with recognised mutations in genes encoding connective tissues [16, 17]. This observation could point towards an underlying aetiological pathway between musculoskeletal hypermobility and idiopathic scoliosis, with excessive bending and rotation of the growing spine contributing to the pathogenesis of AIS.
Delineating the underlying multifactorial pathogenesis of AIS could pave the way to identification of those at risk of both initiation and progression, to guide which individuals need closer monitoring. Given the observation of co-existence of musculoskeletal hypermobility and scoliosis in inherited syndromes, we aimed to systematically review the literature for a relationship between isolated musculoskeletal hypermobility and AIS.
Method
Study selection
The search strategy was constructed to identify studies investigating the relationship between isolated musculoskeletal hypermobility (not as part of an inherited syndrome) and AIS. This was applied to the databases MEDLINE, EMBASE, CINAHL, AHMED and PsychInfo, from inception to September 2021. Forward and backward searches (Google Scholar cited reference search and screening reference lists) were performed on eligible studies.
Search terms for musculoskeletal hypermobility used the corresponding subject heading for each database, and the text words hypermob* or laxity or flexibil* or GJH or GJL or JHS or HSD or "hypermobile Ehlers-Danlos syndrome" or hEDS or EDS-HT or "Ehlers-Danlos type III" or "EDS type III" or "Ehlers-Danlos syndrome type 3" or "EDS type 3". Hypermobile Ehlers-Danlos syndrome was included due to its close clinical overlap with hypermobility spectrum disorder and the absence of a known specific genetic association, making it arguably part of the spectrum of musculoskeletal hypermobility [13]. Search terms for AIS used ‘scoliosis’ as subject heading and text word.
Studies were eligible for inclusion if they assessed the relationship between musculoskeletal hypermobility using any clinical measure of generalised hypermobility, and AIS measured via X-ray or screening methods. Studies assessing musculoskeletal hypermobility or scoliosis as part of an inherited syndrome were excluded. Studies assessing the mobility of a single area of the musculoskeletal system were excluded, as this may only represent localised musculoskeletal hypermobility, and there is often poor correlation between hypermobility in a single area and a diagnosis on the spectrum of generalised musculoskeletal hypermobility [18, 19]. Case reports, case series and conference abstracts were excluded. There was no limit on year of publication or language. The review was registered on PROSPERO on 12/8/21, registration number CRD42021206072.
Records retrieved were screened by title and abstract by CS using Endnote. Full-text articles identified were screened independently by CS and EC based on eligibility criteria. Any discrepancies were resolved through discussion. Characteristics of eligible studies were collected using a standardised Excel spreadsheet (study year, population, sample size, methods of diagnosis of musculoskeletal hypermobility and AIS, curve types, Cobb angles and outcome).
Analysis
Eligible studies were assessed for quality using the Newcastle–Ottawa Scale (NOS) [20] by CS, with uncertainties resolved through discussion with EC. Based on standard classifications of this scale, studies were classified as at high risk of bias if the score was < 5. Those with a score of < 3 were excluded from further analysis. Studies were then assessed for heterogeneity in study design and methods of identification and measurement of hypermobility and AIS, to determine whether meta-analysis was possible. Otherwise, a narrative evidence synthesis was planned. Weighting of studies within any narrative synthesis was performed based on the hierarchy of evidence (study design) and NOS score.
Results
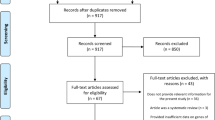
The PRISMA diagram in Fig. 1 shows articles retrieved and screened.
Nineteen studies fulfilled the eligibility criteria (see Table 1); 26 were excluded after full-text review because they either did not include extractable data on hypermobility or AIS, were outside the age range, were conference abstracts or non-relevant reviews or did not examine the association between hypermobility and AIS.
Of the fifteen studies which used a control comparator, or directly correlated musculoskeletal hypermobility and AIS, seven suggested a positive association [21,22,23,24,25,26,27], and eight found no association [28,29,30,31,32], or trends towards a negative association [19, 33, 34].
Of the three studies which reported the prevalence of hypermobility individuals with AIS, one found a high prevalence [35], and two found a prevalence within the reported range for that geographical area [36, 37]. One low-quality study reported the prevalence of scoliosis in a group with joint hypermobility syndrome [38].
Assessment of quality
NOS scores are presented in Table 2. Scores ranged from two to seven, with nine out of the 19 studies scoring < 5 and therefore at high risk of bias. A common aspect which could introduce bias was a lack of adjustment for factors which are recognised as associated with both musculoskeletal hypermobility and AIS, including age, height, BMI and pubertal stage. Only five studies attempted some participant matching or adjustment for confounders [30, 32, 34, 36, 37]. In the case–control studies, only three out of nine attempted to exclude AIS in controls [21, 22, 33].
The study scoring lowest on the NOS (2*) was a descriptive study, characterising features of a cohort with joint hypermobility syndrome referred to a tertiary centre in London, UK [38]. The method for determining scoliosis was not defined, and this was a highly selected population. No further evaluation of this study was undertaken, leaving 18 studies.
Assessment of heterogeneity
To assess appropriateness of undertaking meta-analysis, a comprehensive review of study design and methods of identification and measurement of hypermobility and AIS was undertaken.
Study design
Of the 18 studies assessed, nine were case–control [19, 21,22,23, 27, 28, 33, 34, 37], seven were cross-sectional (five of which were conducted in the general population [25, 26, 29,30,31], and two in dancers and rhythmic gymnasts [24, 32]) and two were cohort studies in individuals with AIS [35, 36].
The studies comprised a total of 17,156 individuals; 15,559 were recruited from the general population (mainly represented by one cross-sectional study of 11,820 individuals [26]), 1,305 were recruited from hospital-based clinics, and 292 individuals were from highly selected populations of dancers and rhythmic gymnasts.
Diagnosis of AIS
The cohort studies in AIS patients [35, 36] and all of the case–control studies used X-ray to define cases, but only three case–control studies attempted to exclude AIS in controls, using X-rays done for other medical reasons [33], or ATR measurement [21, 22].
Of the cross-sectional studies, three used FBT with ATR measurement followed by X-ray if deemed positive [23, 25, 31], three studies used FBT with ATR measurement [26, 32], and one study used visual assessment only (FBT and Magee’s skyline view assessing visible humps or asymmetry) [24]. One cross-sectional study performed spinal X-rays on all participants, raising ethical questions [29].
Diagnosis of musculoskeletal hypermobility
There was wide variation in measures used to diagnose musculoskeletal hypermobility.
Twelve studies used the Beighton score [21, 22, 24, 25, 29,30,31,32, 35,36,37]. Five used the traditional cut-off score of 4/9 [21, 30,31,32, 36], and five used a cut-off of 5/9 [22, 24, 29, 35, 37]. The rationale for using a higher cut-off was justified only by Czaprowski et al. [22] as the sample was comprised of females, who have higher rates of hypermobility. Erkula et al. used a cut-off of 7, but attributed a score of 2 for trunk forward flexion, making the total possible score 10 [25]. Pratelli et al. compared mean Beighton scores rather than applying a cut-off [26].
The remaining studies assessed the mobilities of joints similar to the Beighton score, including Carter and Wilkinson criteria [23, 28], on which the Beighton score was based [39]. Four studies compared a variety of individual tests rather than defining individuals as hypermobile (see Table 1) [19, 27, 33, 34], which may reflect only localised musculoskeletal hypermobility, particularly as two studies found poor correlation between tests within the same individual [19, 33].
Meta-analysis
Due to substantial heterogeneity in study design and methods for identification and measurement of hypermobility and scoliosis, it was not possible to undertake a meta-analysis. Therefore, a narrative synthesis was performed.
Narrative synthesis
Case–control studies
Two high-quality case–control studies found a positive association between musculoskeletal hypermobility and AIS [21, 22], five lower-quality case–control studies found no association [19, 28, 33, 34], and one reported the prevalence of hypermobility as in the reported range for the area among individuals with AIS [10, 37, 40].
The highest quality case–control study was performed by Czaprowski et al., who conducted two similar studies. In the first, they found a higher prevalence of musculoskeletal hypermobility in male and female AIS patients compared to controls (51.4% vs 19.0%, p = 0.00015) [21]. The second study was larger, and only included females [22]. Using a higher cut-off for hypermobility (Beighton score ≥ 5/9), justified by its greater prevalence in females, they also found higher prevalence of hypermobility in AIS patients compared to controls (23.2% vs 13.4%, p = 0.02). Neither study found significant correlation between hypermobility and severity of AIS. The strengths of these studies were confirming similar baseline characteristics in cases and controls, robust inclusion and exclusion criteria, and a reasonable attempt to exclude AIS in controls using ATR measurements.
Four case–control studies, two of which were small (n = 20 [33] and n = 22 [27]), compared a variety of individual tests for musculoskeletal hypermobility, and in general found no differences in hypermobility between those with AIS and controls [19, 27, 33, 34]. Weber found no difference between groups using a total hypermobility score [28]. However, the low threshold is used to define musculoskeletal hypermobility (> 3/14 positive tests), and the inclusion of so many tests impacts the study quality.
Cross-sectional studies carried out in the general population
Of five cross-sectional studies carried out in schoolchildren, three found no association [29,30,31], and two found a positive association between musculoskeletal hypermobility and AIS [25, 26].
The highest quality cross-sectional study found no association between musculoskeletal hypermobility and suspected early spinal curves (measured using back surface topography) with adjustment for posture and BMI (in males OR 0.68 (95% CI 0.3–1.32, p = 0.255), in females OR 0.89 (95% CI 0.50–1.57, p = 0.442)) [30]. The study was designed to sample children just prior to the pubertal growth spurt, a high-risk period for AIS development [41]. Those with known or clearly visible scoliosis were excluded, so we can only conclude that hypermobility was not associated with early small spinal curves in this population.
In contrast, two studies pointed towards a positive association. A large Italian study (n = 11,820) found 2.03% of adolescents had clinical scoliosis (ATR ≥ 5° or hump size ≥ 5 mm) [26]. This group had a higher mean Beighton score compared to those without any clinical spinal curvature (2.41/9 vs 1.96/9). However, these scores are too low to represent generalised musculoskeletal hypermobility, and individuals with inherited syndromes were not excluded, which could have artificially inflated Beighton scores in those with spinal curvature. A Turkish study also found higher Beighton scores in those with radiologically diagnosed AIS, and hypermobile individuals (defined as Beighton score > 7/10) had slightly higher ATR measurements (mean 2.31° vs 1.29°, p = 0.039) [25].
Two studies smaller studies (n = 822 and n = 247), both with relatively high prevalence of radiologically diagnosed AIS, (5.2% and 17.8%) found no association with musculoskeletal hypermobility [29, 31].
Cohort studies in individuals with AIS
Of the two cohort studies in AIS patients, one found a high prevalence of hypermobility (66.6%) [35], and the other found a prevalence within the reported range for the area (25%) [36]. These populations were predominantly female operated patients, with more severe curves. One study found that despite similar curve severity, hypermobile individuals had better surgical outcomes in terms of percentage curve correction [35]. The second larger study (n = 570) found higher Beighton scores were weakly associated with lower Cobb angle (milder curves), which was attenuated but remained after adjustment for age. Being hypermobile did not predict the need for surgical intervention, although lack of trunk hypermobility conferred a 2.5 × increased risk of surgery [36].
Studies in highly selected populations
Three studies were performed in adolescent female rhythmic gymnasts and dancers [23, 24, 32], populations with observed high rates of both musculoskeletal hypermobility and AIS [7, 42].
As expected, there was a high prevalence of musculoskeletal hypermobility (40.9% and 100%) [23, 24]. There was also a higher prevalence of spinal curvature than age-matched controls (30% vs 3.33% using ATR measurement) [32], or females of the same age in that region (12% vs 1.1% using ATR then X-ray) [23]. In a small group of dancers (n = 30), there was no association between musculoskeletal hypermobility and spinal curvature (OR 1.23, 95% CI 0.86–1.75 p = 0.25), although the sample may have been too small to detect an association [32]. There is high risk of confounding in these studies, as rhythmic gymnasts were shorter, lighter, fewer had started menarche, and had reduced lumbar lordosis and thoracic kyphosis, all factors associated with AIS [5, 7, 43]. Equally, dancers with both musculoskeletal hypermobility and spinal curvature had weaker knee musculature, reduced proprioception and anterior balance compared with dancers without either phenotype [24].
Discussion
The literature on the association between isolated musculoskeletal hypermobility and AIS shows varying results. Overall, there is no convincing population-based evidence for an association, although in a group of patients with mild AIS, there was some high-quality evidence for an association [22]. Potential explanations for this disparity could be selection bias or uncontrolled confounding. In selected populations where hypermobility is common, AIS is found more frequently, but again there is high risk of confounding in these studies.
Two cross-sectional studies found higher mean Beighton scores in those with clinically and radiologically diagnosed AIS [25, 26], but these scores were too low to represent a diagnosis of generalised musculoskeletal hypermobility. Hypermobile individuals had slightly higher ATR measurements, which could simply reflect excessive spinal mobility inducing functional reversible curves and therefore higher ATR measurements, which may not translate into progressive scoliotic curves [25]. Indeed, despite correlation with Cobb angle, ATR measurement overestimates the presence of a scoliotic curve in younger adolescents, which could in part be related to higher prevalence of hypermobility [44, 45]. Contrary to this theory, a high-quality cross-sectional study did not find an association between musculoskeletal hypermobility and small early spinal curves after exclusion of those with known scoliosis in an adjusted model [30], although the back surface topography method used here assesses spinal deformity in the coronal plane, as opposed to ATR, a measure of spinal rotation.
The only compelling evidence for an association between musculoskeletal hypermobility comes from two studies by the same authors [21, 22], who found higher rates of musculoskeletal hypermobility in individuals with mild AIS. However, the case–control design inherently risks selection bias, as the AIS group, recruited from a hospital-based clinic, may possess particular confounding characteristics associated with presentation to secondary care, possibly inducing or accentuating any associations.
Studies investigating dancers and rhythmic gymnasts, individuals at the extremes of hypermobility, have found higher rates of AIS [23, 24], although results were not adjusted for potential confounders which were also common in these populations (particularly low BMI and pubertal stage). The results could therefore represent confounding, or hypermobility may be one of a constellation of traits associated with AIS in these populations.
This review highlights important implications for future research into the association between musculoskeletal hypermobility and AIS. Firstly, standardisation of measurement methods would allow replication of results across populations. For musculoskeletal hypermobility, the most commonly used method for diagnosis is the Beighton score, with acceptable inter- and intra-rater reliability, and this should be used by future studies. However, consensus regarding the most appropriate cut-off scores for clinically important musculoskeletal hypermobility are needed [46]. For AIS, the gold-standard would be spinal radiographs in the entire study population, which would entail considerable exposure to ionising radiation, and would therefore be unethical in healthy individuals. The pragmatic use of screening methods to define AIS in a research setting could artificially increase the strength of any association if it overestimates the presence of scoliosis. This is particularly pertinent when investigating musculoskeletal hypermobility, as it is conceivable that excessive spinal mobility could give rise to functional spinal rotation while bending, resulting in a false positive ATR result. Newer imaging methods such as EOS, which reconstructs a 3D model of the spine [47], and techniques for measuring spinal curvature from DXA scans have been developed [48], which confer minimal radiation exposure, and could prove useful in accurately assessing spinal curvature in study populations.
Secondly, future studies must take into account the potential for confounding, particularly age, BMI and pubertal stage, in order to examine the true relationship between musculoskeletal hypermobility and AIS.
Lastly, there were no longitudinal data. It is therefore difficult to determine which curves will progress, a key factor influencing clinical management. Longitudinal data would allow analysis of changes in musculoskeletal hypermobility and curve development through adolescence, and better understanding of their temporal relationship. Determining whether musculoskeletal hypermobility impacts on curve progression could help identify at-risk individuals, and guide frequency of monitoring, or even clinical management if curves of hypermobile individuals behave differently, as hinted at by the finding of greater surgical curve correction in hypermobile individuals [35].
Strengths and limitations
Strengths of this review are the inclusion of studies with a range of measures of generalised musculoskeletal hypermobility and scoliosis, reflecting the available literature, and our ability to include manuscripts written in English, German, Spanish and Italian. Limitations of this review include an inability to carry out a meta-analysis. However, a narrative evidence synthesis was performed, weighted towards studies of the highest quality. Common limitations in design of the eligible studies were identified.
Conclusions
Although there are suggestions of an association between musculoskeletal hypermobility and AIS, there is a paucity of high-quality evidence. Greater understanding of the role of musculoskeletal hypermobility in the pathogenesis of AIS could help to identify factors involved in its initiation and progression and could lead to development of clinical tools to identify individuals most at-risk, to allow more tailored clinical management.
As highlighted by this review, further large-scale prospective studies are required with standardised measures of hypermobility and adequate consideration of potential confounding factors, to clarify the true role of isolated musculoskeletal hypermobility in AIS.
References
Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA (2008) Adolescent idiopathic scoliosis. Lancet 371(9623):1527–1537
Clark EM, Tobias JH, Fairbank J (2016) The impact of small spinal curves in adolescents who have not presented to secondary care: a population-based cohort study. Spine (Phila Pa 1976) 41(10):E611–E617
Tones M, Moss N, Polly DW Jr (2006) A review of quality of life and psychosocial issues in scoliosis. Spine (Phila Pa 1976) 31(26):3027–3038
Johnston CE, Richards BS, Sucato DJ, Bridwell KH, Lenke LG, Erickson M (2011) Correlation of preoperative deformity magnitude and pulmonary function tests in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 36(14):1096–1102
Clark EM, Taylor HJ, Harding I, Hutchinson J, Nelson I, Deanfield JE et al (2014) Association between components of body composition and scoliosis: a prospective cohort study reporting differences identifiable before the onset of scoliosis. J Bone Miner Res 29(8):1729–1736
Shohat M, Shohat T, Nitzan M, Mimouni M, Kedem R, Danon YL (1988) Growth and ethnicity in scoliosis. Acta Orthop Scand 59(3):310–313
Warren MP, Brooks-Gunn J, Hamilton LH, Warren LF, Hamilton WG (1986) Scoliosis and fractures in young ballet dancers. Relation to delayed menarche and secondary amenorrhea. New Engl J Med 314(21):1348–1353
Negrini S, Donzelli S, Aulisa AG, Czaprowski D, Schreiber S, de Mauroy JC et al (2018) 2016 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord 13:3
Aartun E, Hartvigsen J, Hestbaek L (2016) Validity of commonly used clinical tests to diagnose and screen for spinal pain in adolescents: a school-based cohort study in 1300 Danes aged 11–15 years. J Manipulative Physiol Ther 39(2):76–87
de Inocencio AJ, Ocaña Casas I, Benito OL (2004) Laxitud articular: prevalencia y relación con dolor musculosquelético. Anal Pediatr 61(2):162–166
Subramanyam V, Janaki KV (1996) Joint hypermobility in south Indian children. Indian Pediatr 33(9):771–772
Remvig L, Jensen DV, Ward RC (2007) Epidemiology of general joint hypermobility and basis for the proposed criteria for benign joint hypermobility syndrome: review of the literature. J Rheumatol 34(4):804–809
Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H et al (2017) Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet C Semin Med Genet 175(1):48–69
Beighton P, Solomon L, Soskolne CL (1973) Articular mobility in an African population. Ann Rheum Dis 32(5):413–418
Clinch J, Deere K, Sayers A, Palmer S, Riddoch C, Tobias JH et al (2011) Epidemiology of generalized joint laxity (hypermobility) in fourteen-year-old children from the UK: a population-based evaluation. Arthritis Rheum 63(9):2819–2827
Arponen H, Mäkitie O, Waltimo-Sirén J (2014) Association between joint hypermobility, scoliosis, and cranial base anomalies in paediatric osteogenesis imperfecta patients: a retrospective cross-sectional study. BMC Musculoskelet Disord 15:428
Meester JAN, Verstraeten A, Schepers D, Alaerts M, Van Laer L, Loeys BL (2017) Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann Cardiothorac Surg 6(6):582–594
Malek S, Reinhold EJ, Pearce GS (2021) The Beighton Score as a measure of generalised joint hypermobility. Rheumatol Int 41(10):1707–1716
Mattson G, Haderspeck-Grib K, Schultz A, Nachemson A (1983) Joint flexibilities in structurally normal girls and girls with idiopathic scoliosis. J Orthop Res 1(1):57–62
Wells G SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Czaprowski D, Kotwicki T, Pawlowska P, Stolinski L (2011) Joint hypermobility in children with idiopathic scoliosis: SOSORT award 2011 winner. Scoliosis 6(1):10
Czaprowski D (2014) Generalised joint hypermobility in caucasian girls with idiopathic scoliosis: relation with age, curve size, and curve pattern. Sci World J 2014:1–6
Tanchev PI, Dzherov AD, Parushev AD, Dikov DM, Todorov MB (2000) Scoliosis in rhythmic gymnasts. Spine 25(11):1367–1372
Steinberg N, Tenenbaum S, Zeev A, Pantanowitz M, Waddington G, Dar G et al (2021) Generalized joint hypermobility, scoliosis, patellofemoral pain, and physical abilities in young dancers. BMC Musculoskelet Disord 22(1):1–11
Erkula G, Kiter AE, Kilic BA, Er E, Demirkan F, Sponseller PD (2005) The relation of joint laxity and trunk rotation. J Pediatr Orthop B 14(1):38–41
Pratelli E, Apicella L, Bertaccini B, Petrocelli A, Petrai V, Carulli C et al (2020) Results of a vertebral deformity screening in the students of the district of Florence (Tuscany Region, Central Italy) [Italian]. Epidemiol Prevenzione 44(2–3):154–161
Kobesova A, Drdakova L, Andel R, Kolar P (2013) Cerebellar function and hypermobility in patients with idiopathic scoliosis. Int Musculoskel Med 35(3):99–105
Weber M (1980) Hypermobility and scoliosis [German]. Orthopad Praxis 16(2):117–119
Farro-Uceda L, Tapia-Egoavil R, Valverde-Tarazona C, Bautista-Chirinos L, Amaya-Solis K (2016) Relación entre hiperlaxitud articular, dismetría de miembros inferiores y control postural con los trastornos posturales. Rev Med Herediana 27(4):216–222
Dolphens M, Vleeming A, Castelein R, Vanderstraeten G, Schlösser T, Plasschaert F et al (2018) Coronal plane trunk asymmetry is associated with whole-body sagittal alignment in healthy young adolescents before pubertal peak growth. Eur Spine J 27(2):448–457
Bozkurt S, Kayalar G, Tezel N, Güler T, Kesikburun B, Denizli M et al (2019) Hypermobility frequency in school children: relationship with idiopathic scoliosis, age, sex and musculoskeletal problems. Arch Rheumatol 34(3):268–273
Longworth B, Fary R, Hopper D (2014) Prevalence and predictors of adolescent idiopathic scoliosis in adolescent ballet dancers. Arch Phys Med Rehabil 95(9):1725–1730
Veldhuizen AG, Scholten PJM (1990) Flexibility in structurally normal young females and in young females with idiopathic scoliosis. Clin Biomech 5(2):117–126
Fuller BJ, Bishop PA, Mansfield ER, Smith JF (1991) Strength, muscle symmetry, and flexibility in young female idiopathic scoliotics. J Orthop Sports Phys Ther 14(4):144–148
Hasankhani EG, Omidi-Kashani F (2012) Generalized ligamentous laxity; a parameter should not preoperative planning of adolescent idiopathic scoliosis. Iran Red Crescent Med J 14(11):707–709
Haller G, Zabriskie H, Spehar S, Kuensting T, Bledsoe X, Syed A et al (2018) Lack of joint hypermobility increases the risk of surgery in adolescent idiopathic scoliosis. J Pediatr Orthopaed B 27(2):152–158
Fernandez-Bermejo E, Garcia-Jimenez MA, Fernandez-Palomeque C, Munuera L, Fernandez-Bermejo E, García-Jiménez MA et al (1993) Adolescent idiopathic scoliosis and joint laxity. A study with somatosensory evoked potentials. Spine 18(7):918–922
Adib N, Davies K, Grahame R, Woo P, Murray KJ (2005) Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology. 44(6):744–750
Carter C, Wilkinson J (1964) Persistent joint laxity and congenital dislocation of the hip. J Bone Joint Surg Br 46:40–45
Zurita Ortega F, Ruiz Rodríguez L, Martínez Martínez A, Fernández Sánchez M, Rodríguez Paiz C, López LR (2010) Hiperlaxity ligamentous (Beighton test) in the 8 to 12 years of age school population in the province of Granada. Reumatol Clin 6(1):5–10
Cheng JC, Castelein RM, Chu WC, Danielsson AJ, Dobbs MB, Grivas TB et al (2015) Adolescent idiopathic scoliosis. Nat Rev Dis Primers 1:15030
McCormack M, Briggs J, Hakim A, Grahame R (2004) Joint laxity and the benign joint hypermobility syndrome in student and professional ballet dancers. J Rheumatol 31(1):173–178
Castelein RM, Veraart B (1992) Idiopathic scoliosis: prognostic value of the profile. Eur Spine J 1(3):167–169
Bunnell WP (2005) Selective screening for scoliosis. Clin Orthop Relat Res 434:40–45
Grivas TB, Vasiliadis ES, Mihas C, Savvidou O (2007) The effect of growth on the correlation between the spinal and rib cage deformity: implications on idiopathic scoliosis pathogenesis. Scoliosis 2:11
Juul-Kristensen B, Schmedling K, Rombaut L, Lund H, Engelbert RH (2017) Measurement properties of clinical assessment methods for classifying generalized joint hypermobility—a systematic review. Am J Med Genet C Semin Med Genet 175(1):116–147
Deschênes S, Charron G, Beaudoin G, Labelle H, Dubois J, Miron MC et al (2010) Diagnostic imaging of spinal deformities: reducing patients radiation dose with a new slot-scanning X-ray imager. Spine (Phila Pa 1976) 35(9):989–994
Jamaludin A, Fairbank J, Harding I, Kadir T, Peters TJ, Zisserman A et al (2020) Identifying scoliosis in population-based cohorts: automation of a validated method based on total body dual energy X-ray absorptiometry scans. Calcif Tissue Int 106(4):378–385
Acknowledgements
This research was supported by the Elizabeth Blackwell Institute, University of Bristol and funded in whole, or in part, by the Wellcome Trust (grant number 204813/Z/16/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Funding
This research was supported by the Elizabeth Blackwell Institute, University of Bristol and funded in whole, or in part, by the Wellcome Trust (grant number 204813/Z/16/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
EC had the idea for the review. CS performed the literature search and performed data extraction. EC and CS performed data analysis, drafted and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This systematic review did not require ethical approval.
Informed consent
This systematic review did not require the application of informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shere, C., Clark, E.M. Systematic review of the association between isolated musculoskeletal hypermobility and adolescent idiopathic scoliosis. Arch Orthop Trauma Surg 143, 3055–3076 (2023). https://doi.org/10.1007/s00402-022-04508-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04508-z